Abstract
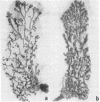
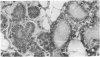
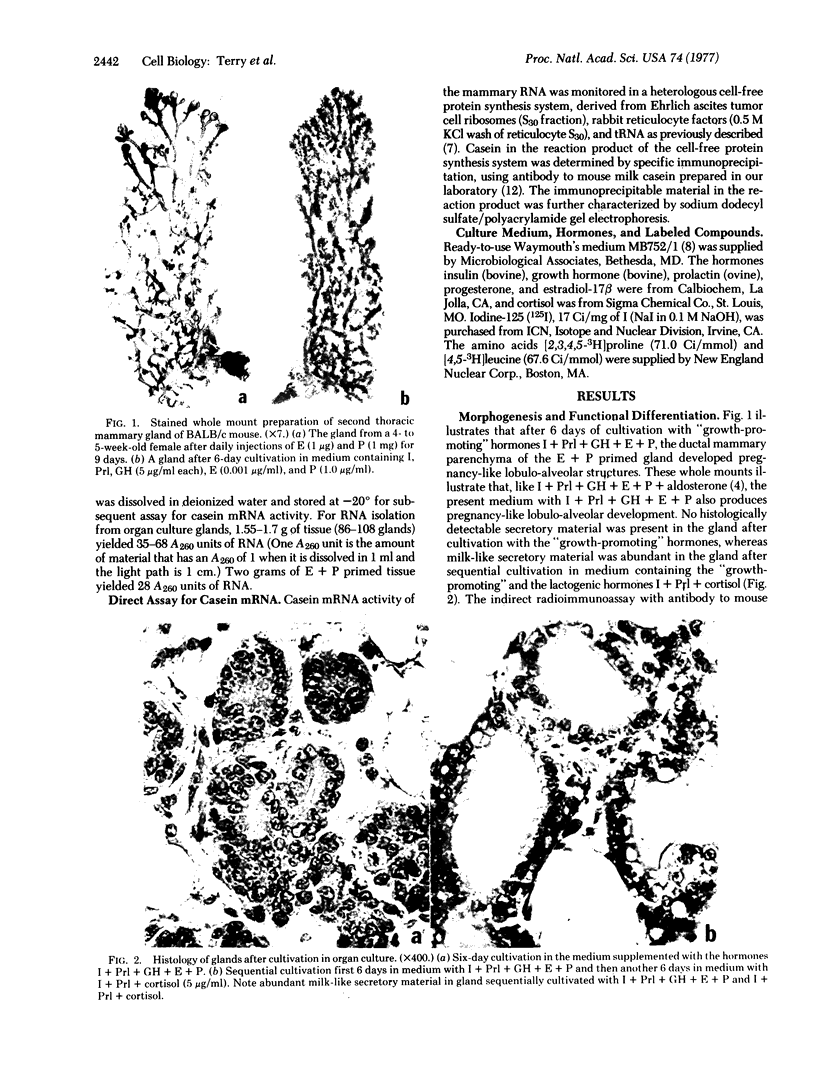
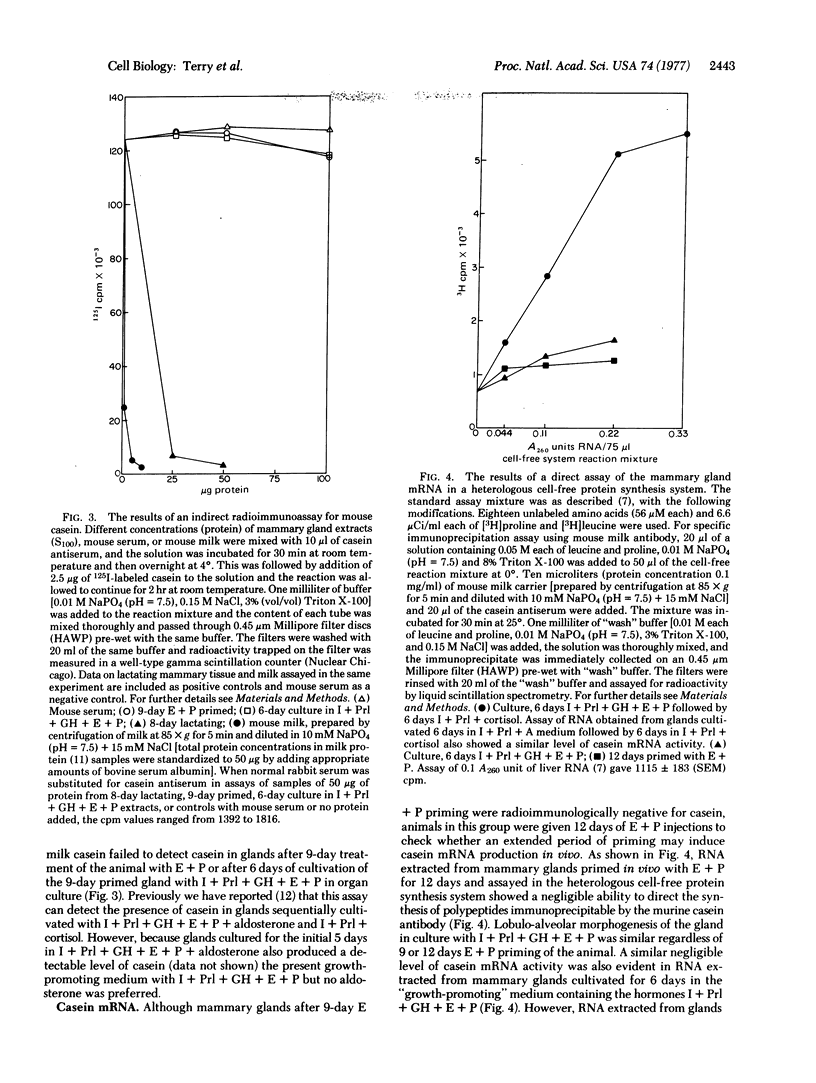
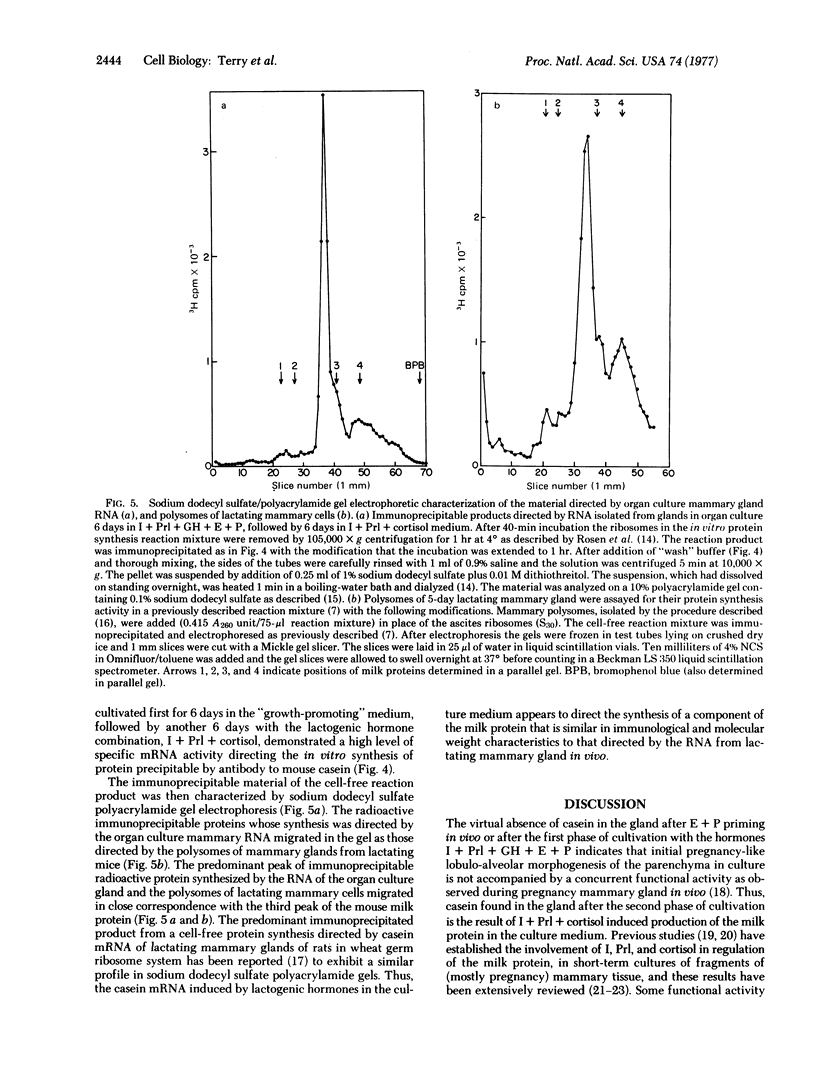
The whole second thoracic mammary gland of estradiol-17β + progesterone primed 3- to 4-week-old BALB/c female mice was induced to pregnancy-like lobulo-alveolar morphogenesis after 6-day cultivation in a serum-free culture medium containing a “growth promoting” hormone mixture, insulin + prolactin + growth hormone (somatotropin) + estradiol + progesterone. No radioimmunologically detectable casein was present in these glands. Subsequent cultivation for another 6 days in a “lactogenic” medium with the hormones insulin + prolactin + cortisol produced abundant milk-like secretory material in the alveolar lumen. RNA of the mammary gland after estradiol + progesterone priming or cultivation in the “growth-promoting” medium failed to show a measurable amount of casein mRNA activity when assayed in a cell-free protein synthesis system derived from Ehrlich ascites ribosomes, rabbit reticulocyte factors, and tRNA. However, the glands sequentially cultivated in the “growth-promoting” and the “lactogenic” media showed a high level of casein mRNA activity in the heterologous cell-free protein synthesis system. Sodium dodecyl sulfate/polyacrylamide gel electrophoretic characteristics of the immunoprecipitable (by antibody to mouse milk casein) polypeptides directed by the mammary RNA induced in organ culture medium containing the lactogenic hormones were similar to the characteristics of the polypeptides directed by mammary polysomes of lactating mice. These results demonstrate hormonal induction of a specific mRNA in a sequential two-step culture of an entire organ in a serum-free chemically defined medium.
Keywords: indirect radioimmunoassay, ascites ribosome protein synthesis system, radioimmunoprecipitation, sodium dodecyl sulfate-polyacrylamide gels
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee M. R. Responses of mammary cells to hormones. Int Rev Cytol. 1976;47:1–97. doi: 10.1016/s0074-7696(08)60086-8. [DOI] [PubMed] [Google Scholar]
- Banerjee M. R., Wood B. G., Washburn L. L. Chemical carcinogen-induced alveolar nodules in organ culture of mouse mammary gland. J Natl Cancer Inst. 1974 Nov;53(5):1387–1393. doi: 10.1093/jnci/53.5.1387. [DOI] [PubMed] [Google Scholar]
- Ichinose R. R., Nandi S. Influence of hormones on lobulo-alveolar differentiation of mouse mammary glands in vitro. J Endocrinol. 1966 Aug;35(4):331–340. doi: 10.1677/joe.0.0350331. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mena F., Enjalbert A., Carbonell L., Priam M., Kordan C. Effect of suckling on plasma prolactin and hypothalamic monoamine levels in the rat. Endocrinology. 1976 Aug;99(2):445–451. doi: 10.1210/endo-99-2-445. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. S., Washburn L. L., Banerjee M. R. Role of insulin as a "permissive" hormone in mammary gland development. Nature. 1973 Nov 16;246(5429):159–160. doi: 10.1038/246159a0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Moore P. B., Mulvihill E. R. A significant lag in the induction of ovalbumin messenger RNA by steroid hormones: a receptor translocation hypothesis. Cell. 1976 Aug;8(4):557–572. doi: 10.1016/0092-8674(76)90224-5. [DOI] [PubMed] [Google Scholar]
- RIVERA E. M., BERN H. A. Influence of insulin on maintenance and secretory stimulation of mouse mammary tissues by hormones in organ-culture. Endocrinology. 1961 Aug;69:340–353. doi: 10.1210/endo-69-2-340. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Comstock J. P. Regulation of casein messenger RNA during the development of the rat mammary gland. Biochemistry. 1975 Jul;14(13):2895–2903. doi: 10.1021/bi00684a016. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Holder J. W., Means A. R., O'Malley B. W. Preparation and preliminary characterization of purified ovalbumin messenger RNA from the hen oviduct. Biochemistry. 1975 Jan 14;14(1):69–78. doi: 10.1021/bi00672a012. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., McKnight G. S., Shapiro D. J., Sullivan D., Palacios R. Hormonal regulation of ovalbumin synthesis in the chick oviduct. Recent Prog Horm Res. 1975;31:175–211. doi: 10.1016/b978-0-12-571131-9.50009-8. [DOI] [PubMed] [Google Scholar]
- Terry P. M., Ball E. M., Ganguly R., Banerjee M. R. An indirect radioimmunoassay for mouse casein using 125I-labeled antigen. J Immunol Methods. 1975 Dec;9(2):123–134. doi: 10.1016/0022-1759(75)90102-7. [DOI] [PubMed] [Google Scholar]
- Terry P. M., Ganguly R., Ball E. M., Banerjee M. R. Murine mammary gland RNA directed synthesis of casein in a heterologous cell-free protein synthesis system. Cell Differ. 1975 May;4(2):113–122. doi: 10.1016/0045-6039(75)90023-8. [DOI] [PubMed] [Google Scholar]
- Topper Y. J. Multiple hormone interactions in the development of mammary gland in vitro. Recent Prog Horm Res. 1970;26:287–308. doi: 10.1016/b978-0-12-571126-5.50011-x. [DOI] [PubMed] [Google Scholar]
- Turkington R. W., Majumder G. C., Kadoama N., MacIndoe J. H., Frantz W. L. Hormonal regulation of gene expression in mammary cells. Recent Prog Horm Res. 1973;29:417–455. doi: 10.1016/b978-0-12-571129-6.50015-4. [DOI] [PubMed] [Google Scholar]
- WAYMOUTH C. Rapid proliferation of sublines of NCTC clone 929 (strain L) mouse cells in a simple chemically defined medium (MB 752/1). J Natl Cancer Inst. 1959 May;22(5):1003–1017. doi: 10.1093/jnci/22.5.1003. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wood B. G., Washburn L. L., Mukherjee A. S., Banerjee M. R. Hormonal regulation of lobulo-alveolar growth, functional differentiation and regression of whole mouse mammary gland in organ culture. J Endocrinol. 1975 Apr;65(1):1–6. doi: 10.1677/joe.0.0650001. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]