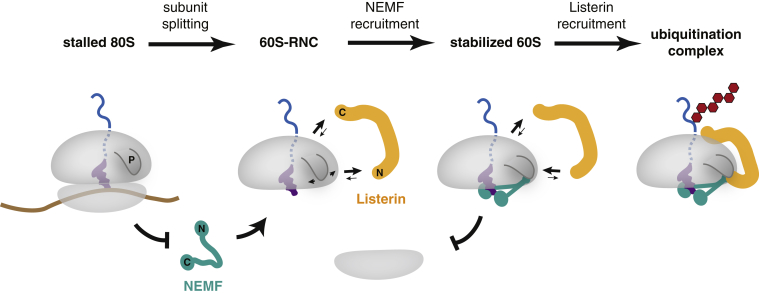
Figure 7.
Working Model for Step-Wise Assembly of RQC Ubiquitination Complex
Translationally stalled 80S ribosomes, which are inaccessible to both Listerin (orange) and NEMF (teal) binding, are split into subunits by the factors Pelota, Hbs1, and ABCE1 (not displayed). Ribosome splitting exposes the peptidyl tRNA of a trapped nascent chain within the 60S subunit. At this stage, Listerin can potentially bind, but is competed by 40S reassociation and a dynamic P stalk (P). By contrast, NEMF specifically recognizes and binds the peptidyl tRNA-60S interface via its globular N- and C-terminal lobes. Upon binding the 60S-tRNA interface, the coiled coil and M-domain of NEMF bind and stabilize the P stalk in a defined position. This generates an improved binding site for the N terminus of Listerin between NEMF and the 60S, facilitating docking of its C-terminal RWD domain. The ribosome-bound RWD domain positions the ligase domain at the nascent chain exit tunnel, leading to a productive ubiquitination complex.