Abstract
Objectives
Biofilms at tooth-restoration margins can produce acids and cause secondary caries. A protein-repellent adhesive resin can potentially inhibition bacteria attachment and biofilm growth. However, there has been no report on protein-repellent dental resins. The objectives of this study were to develop a protein-repellent bonding agent incorporating 2-methacryloyloxyethyl phosphorylcholine (MPC), and to investigate its resistance to protein adsorption and biofilm growth for the first time.
Methods
MPC was incorporated into Scotchbond Multi-Purpose (SBMP) at 0%, 3.75%, 7.5%, 11.25%, and 15% by mass. Extracted human teeth were used to measure dentin shear bond strengths. Protein adsorption onto resins was determined by a micro bicinchoninic acid (BCA) method. A dental plaque microcosm biofilm model with human saliva as inoculum was used to measure biofilm metabolic activity and colony-forming unit (CFU) counts.
Results
Adding 7.5% MPC into primer and adhesive did not decrease the dentin bond strength, compared to control (p > 0.1). Incorporation of 7.5% of MPC achieved the lowest protein adsorption, which was 20-fold less than that of control. Incorporation of 7.5% of MPC greatly reduced bacterial adhesion, yielding biofilm total microorganism, total streptococci, and mutans streptococci CFU that were an order of magnitude less than control.
Conclusions
A protein-repellent dental adhesive resin was developed for the first time. Incorporation of MPC into primer and adhesive at 7.5% by mass greatly reduced the protein adsorption and bacterial adhesion, without compromising the dentin bond strength. The novel protein-repellent primer and adhesive are promising to inhibit biofilm formation and acid production, to protect the tooth-restoration margins and prevent secondary caries.
Keywords: Protein repellent, bacteria repellent, dental adhesive, dentin bond strength, human saliva microcosm biofilm, caries inhibition
1. Introduction
Dental caries is a prevalent disease which results in a heavy financial burden worldwide.1,2 Nearly 200 million tooth cavity restorations are performed in the United States each year.3 The demand for tooth restorations is increasing rapidly with an aging population and increased tooth retention in seniors.4 The fact that more teeth are retained into an elderly age has resulted in more occurrences of dental caries.5 Composites are the principal material for cavity restorations due to their excellent aesthetics and direct-filling capability.6,7 The compositions and properties of resin matrices, fillers and composites have been significantly improved in previous studies.8–13 Nonetheless, approximately half of all restorations fail within 10 years, and the replacement of failed restorations accounts for more than half of all restorations performed.14 Previous studies showed that dental resins in vivo tend to accumulate more biofilms and plaques than other restorative materials.15,16 Furthermore, microgaps can be observed at the tooth-restoration interfaces.17,18 Microleakage can occur and biofilms at the restoration margins can produce acids and cause secondary caries. Secondary caries has been suggested in previous studies as a primary reason for restoration failure.7,19,20
Bonding agents enable the composite restoration to be adhered to the tooth structure.21–23 Extensive studies have been performed to improve, characterize and understand enamel and dentin bonding.24,25 It is beneficial for the bonding agent to be antibacterial, to combat biofilms and secondary caries at the margins. Efforts have been made to develop antibacterial primers and adhesives that could kill bacteria,26–31 and several different compositions of quaternary ammonium methacrylates (QAMs) were synthesized.26–31 For example, 12-methacryloyloxydodecyl-pyridinium bromide (MDPB) was incorporated into primer and adhesive to combat bacteria and biofilm growth.26,27 Recently, a quaternary ammonium dimethacrylate (QADM) was synthesized and incorporated into primer28 and adhesive29 which reduced biofilm viability and acid production.
In the oral environment with salivary flow, a clean dental resin is quickly coated with a salivary pellicle that comprises of a layer of selectively adsorbed salivary proteins.32 It is through this protein layer that oral bacteria attach to the resin and to tooth surfaces.33,34 The adherence of early colonizers, for example, mutans streptococcus, to the salivary pellicle is an initial step in biofilm formation.33,34 Biofilm formation is the source of infection and a prerequisite for the occurrence of dental caries.35 Therefore, it would be highly desirable to develop a new adhesive resin that can repel proteins, to inhibit protein adsorption and hence bacterial adhesion at the tooth-restoration margins and at the eventual microgaps in the margins. A previous study immobilized a protein-repellent material, poly(ethylene glycol) (PEG) and two pyridinium group-containing methacrylate monomers, to silicon wafer surfaces to investigate the influence of prior protein adsorption on bactericidal activity.36 The results showed that the PEG-modified surfaces had substantially less adsorbed proteins.36 However, to date there has been no report on dental adhesive resins that possess protein-repellent capability.
It has been demonstrated that hydrophilic material surfaces are usually more resistant to protein adsorption and bacterial adhesion than hydrophobic surfaces.37,38 2-methacryloyloxyethyl phosphorylcholine (MPC) is a methacrylate with a phospholipid polar group in the side chain, and is one of the most common biocompatible and hydrophilic biomedical polymers.39 MPC shows excellent resistance to protein adsorption and bacterial adhesion,40,41 and has been used in artificial blood vessels,42 artificial hip joints,43 and microfluidic devices.44 The MPC polymer coating renders the surfaces extremely hydrophilic, prevents the adhesion of proteins, and inhibits the adhesion of bacteria.39–41 Various medical devices using MPC have already been developed and clinically used with the approval of the United States Food and Drug Administration.45,46 Previous study evaluated the durability and antiadhesive action of MPC grafting on an acrylic resin denture base material.47 The results demonstrated that graft polymerization of MPC on denture surfaces contributed to the durability of the coating and prevented microbial retention. However, there has been no report on the application of MPC to the dentin bonding agents.
Accordingly, the objectives of this study were to develop protein-repellent dental adhesive resin incorporating MPC and to investigate the resistance of protein adsorption and oral bacterial adherence for the first time. It was hypothesized that: (1) Incorporating MPC into primer and adhesive would not compromise the dentin bond strength; (2) MPC-containing primer and adhesive would have much less protein adsorption than that of commercial bonding agent control; and (3) protein-repellent MPC-containing primer and adhesive would greatly reduce biofilm growth than commercial bonding agent control.
2. Materials and methods
2.1. Fabrication of protein-repellent bonding agent
Scotchbond Multi-Purpose (SBMP, 3M, St. Paul, MN) was used as the parent system. According to the manufacturer, SBMP adhesive contained 60–70% of bisphenol A diglycidyl methacrylate (BisGMA) and 30–40% of 2-hydroxyethyl methacrylate (HEMA), tertiary amines and photo-initiator. SBMP primer contained 35–45% of HEMA, 10–20% of a copolymer of acrylic and itaconic acids, and 40–50% water.
MPC (Sigma-Aldrich, St. Louis, MO) was commercially available which was synthesized via a method reported by Ishihara et al.39 The MPC powder was mixed with SBMP primer at MPC/(SBMP primer + MPC) mass fractions of 0%, 3.75%, 7.5%, 11.25% and 15%. The 7.5% was selected following previous studies.43,44 The other mass fractions enabled the investigation of the relationship between MPC mass fraction and protein-repellent efficacy in a dental resin. MPC mass fractions higher than 15% were not included due to a decrease in the dentin bond strength. Each batch of primer was magnetically-stirred with a bar at a speed of 150 rpm (Bellco Glass, Vineland, NJ) for 24 h to completely dissolve the MPC in the SBMP primer. Similarly, MPC was mixed into SBMP adhesive at the same mass fractions. Hence, five bonding agents were tested:
SBMP primer and adhesive control;
SBMP primer + 3.75% MPC, SBMP adhesive + 3.75% MPC (termed “3.75% MPC”);
SBMP primer + 7.5% MPC, SBMP adhesive + 7.5% MPC (“7.5% MPC”);
SBMP primer + 11.25% MPC, SBMP adhesive + 11.25% MPC (“11.25% MPC”);
SBMP primer + 15% MPC, SBMP adhesive + 15% MPC (“15% MPC”).
For protein adsorption and biofilm testing, resin disks were prepared following previous studies.27,48 Briefly, the cover of a 96-well plate was used as molds. 10 μL of a primer was placed in the bottom of each dent. After drying with a stream of air, 20 μL of adhesive was applied to the dent and photo-polymerized for 30 s using a quartz-tungsten-halogen light-curing unit (Demetron VCL 401, Demetron, CA) with output intensity of 600 mW/cm2, using a mylar strip covering to obtain a disk of approximately 8 mm in diameter and 0.5 mm in thickness. The cured disks were immersed in 200 mL of distilled water and magnetically-stirred with a bar at a speed of 100 rpm for 1 h to remove any uncured monomers, following a previous study.26
2.2. Dentine shear bond testing
Extracted caries-free human molars were sawed to remove the crowns (Isomet, Buehler, Lake Bluff, IL), then ground perpendicularly to the longitudinal axis on 320 grit SiC paper until occlusal enamel was completely removed.28,29 The dentin surface was etched for 15 s and rinsed with water.49 A primer was applied, and the solvent was removed with an air stream. An adhesive was applied and light-cured for 10 s. A stainless-steel iris, having a central opening with a diameter of 4 mm and a thickness of 1.5 mm, was held against the adhesive-treated dentin surface. The central opening was filled with a composite (TPH, Caulk/Dentsply, Milford, DE) and light-cured for 60 s.49
The bonded specimens were stored in distilled water at 37°C for 24 h. Dentin shear bond strength, SD, was measured as previously described.28,49 Briefly, a chisel was held parallel to the composite-dentine interface and loaded via a Universal Testing Machine (MTS, Eden Prairie, MN) at 0.5 mm/min until the composite-dentine bond failed. SD was calculated as: SD = 4P/(πd2), where P is the load at failure, and d is the diameter of the composite.28,49 Ten teeth were tested for each of the aforementioned five group.
2.3. Characterization of protein adsorption by micro bicinchoninic acid method
The amount of protein adsorbed on resin disks was determined by the micro bicinchoninic acid (BCA) method.43,44,47 Each disk was immersed in phosphate buffered saline (PBS) for 2 h before immersing in 4.5 g/L bovine serum albumin (BSA) (Sigma-Aldrich) solutions at 37 °C for 2 h.43,44 The disks then were rinsed with fresh PBS by stirring method (300 rpm for 5 min). The adsorbed protein was detached in sodium dodecyl sulfate (SDS) 1 wt% in PBS by sonication for 20 min. A protein analysis kit (micro BCA protein assay kit, Fisher Scientific, Pittsburgh, PA) was used to determine the BSA concentration in the SDS solution. From the concentration of protein, the amount of protein adsorbed on the resin disk surface was calculated.43,44 Six disks were evaluated for each group.
2.4. Dental plaque microcosm biofilm model
A dental plaque microcosm biofilm model using human saliva was used to test the protein-repellent resins.28,29 Saliva is ideal for growing microcosm biofilms in vitro, with the advantage of maintaining much of the complexity and heterogeneity of the dental plaque in vivo.50 Saliva was collected from ten healthy donors having natural dentition without active caries, and not having used antibiotics within the preceding 3 months. The donors did not brush teeth for 24 h and abstained from food and drink intake for 2 h prior to donating saliva. Stimulated saliva was collected during parafilm chewing and was kept on ice. An equal volume of saliva from each of the ten donors was combined to form the saliva sample. The saliva was diluted in sterile glycerol to a saliva concentration of 70%, and stored at −80 °C for subsequent use.51
The saliva-glycerol stock was added, with 1:50 dilution, into the growth medium as inoculum. The growth medium contained mucin (type II, porcine, gastric) at a concentration of 2.5 g/L; bacteriological peptone, 2.0 g/L; tryptone, 2.0 g/L; yeast extract, 1.0 g/L; NaCl, 0.35 g/L, KCl, 0.2 g/L; CaCl2, 0.2 g/L; cysteine hydrochloride, 0.1 g/L; hemin, 0.001 g/L; vitamin K1, 0.0002 g/L, at pH 7.52 The resin disks were sterilized in ethylene oxide (Anprolene AN 74i, Andersen, Haw River, NC). Then, 1.5 mL of inoculum was added to each well of 24-well plates containing a resin disk, and incubated at 37 °C in 5% CO2 for 8 h. Then, the disks were transferred to new 24-well plates filled with fresh medium and incubated. After 16 h, the disks were transferred to new 24-well plates with fresh medium and incubated for 24 h. This totaled 48 h of incubation, which was adequate to form plaque microcosm biofilms as shown in a previous study.51
2.5. Live/dead assay
Resin disks with 2-day biofilms were washed with PBS and stained using the BacLight live/dead kit (Molecular Probes, Eugene, OR).28,29 Live bacteria were stained with Syto 9 to produce a green fluorescence. Bacteria with compromised membranes were stained with propidium iodide to produce a red fluorescence. The stained disks were examined using an inverted epifluorescence microscope (Eclipse TE2000-S, Nikon, Melville, NY). Six disks were evaluated for each group. Three randomly chosen fields of view were photographed from each disk, yielding a total of 18 images for each group.
2.6. MTT assay of metabolic activity
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was used to examine the metabolic activity of the 2-day biofilms on resin disks.28,29 MTT is a colorimetric assay that measures the enzymatic reduction of MTT, a yellow tetrazole, to formazan. Disks with 2-day biofilms (n = 6) were transferred to a new 24-well plate, then 1 mL of MTT dye (0.5 mg/mL MTT in PBS) was added to each well and incubated at 37°C in 5% CO2 for 1 h. During this process, metabolically active bacteria reduced the MTT to purple formazan. After 1 h, the disks were transferred to a new 24-well plate, 1 mL of dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals. The plate was incubated for 20 min with gentle mixing at room temperature in the dark. Then, 200 μL of the DMSO solution from each well was collected, and its absorbance at 540 nm was measured via a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA). A higher absorbance is related to a higher formazan concentration, which indicates a higher metabolic activity in the biofilm on the disk.28,29
2.7. Colony forming unit (CFU) counts
Resin disks with 2-day biofilms were rinsed with PBS to remove loose bacteria.28,29 Then the disks were transferred into tubes with 2 mL PBS, and the biofilms were harvested by sonication (3510RMTH, Branson, Danbury, CT) for 5 min, followed by vortexing at 2400 rpm for 30 s using a vortex mixer (Fisher Scientific).28,29 Three types of agar plates were examined: First, tryptic soy blood plates were used to determine total micro-organisms.51 Second, mitis salivarius agar (MSA) culture plates plus 15% sucrose were used to determine total streptococci.53 This is because MSA contains selective agents including crystal violet, potassium tellurite and trypan blue, which inhibit most Gram-negative bacilli and most Gram-positive bacteria except streptococci, thus enabling the streptococci to grow.53 Third, cariogenic mutans streptococci is known to be resistant to bacitracin, and this property is used to isolate mutans streptococci from the highly heterogeneous oral microflora.51 Therefore, MSA plus 0.2 units of bacitracin per mL was used to determine mutans streptococci.51 The purpose of measuring these three types of CFU counts was to provide bacterial adhesion and viability on not only the total microorganisms, but also mutans streptococci, in the dental plaque microcosm biofilms. The mutans streptococci group consists of mutans streptococcus and sobrinus streptococcus, both species playing a key role in dental caries. The bacterial suspensions were serially diluted, spread onto agar plates and incubated at 37 °C in 5% CO2 for 24 h. The number of colonies that grew was counted and used, along with the dilution factor, to calculate total CFU counts on each disk.28,29
2.8. Statistical analysis
All data were first checked for normal distribution with the Kolmogorov-Smirnov test. One-way and two-way analyses of variance (ANOVA) were performed to detect the significant effects of the variables. Tukey’s multiple comparison test was used to compare the data at a p value of 0.05.
3. Results
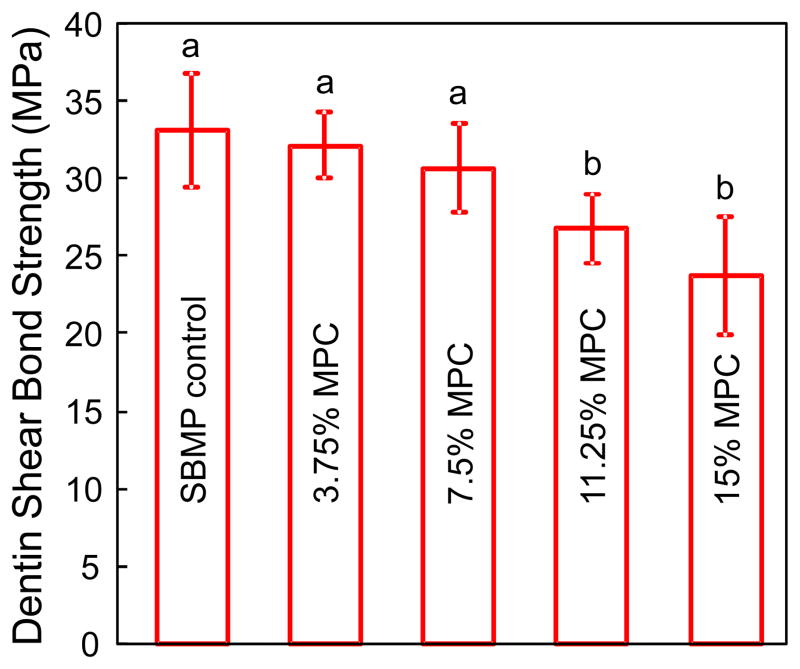
Fig. 1 plots the dentin shear bond strength results (mean ± sd; n = 10). Increasing the MPC mass fraction to 11.25% and 15% MPC caused a decrease in dentin bond strength (p < 0.05). However, the SBMP bonding agents with 3.75% and 7.5% MPC had similar bond strengths to control (p > 0.1). At 7.5% MPC, the dentin bond strength was (30 ± 2.8) MPa, not significantly different from the (33 ± 3.6) MPa of SBMP control (p > 0.1).
Fig. 1.
Dentin bond strength results (mean ± sd; n = 10). Dissimilar letters indicate values that are significantly different from each other (p < 0.05). Bonding agent containing 7.5% MPC had a bond strength similar to that of control without MPC (p > 0.1).
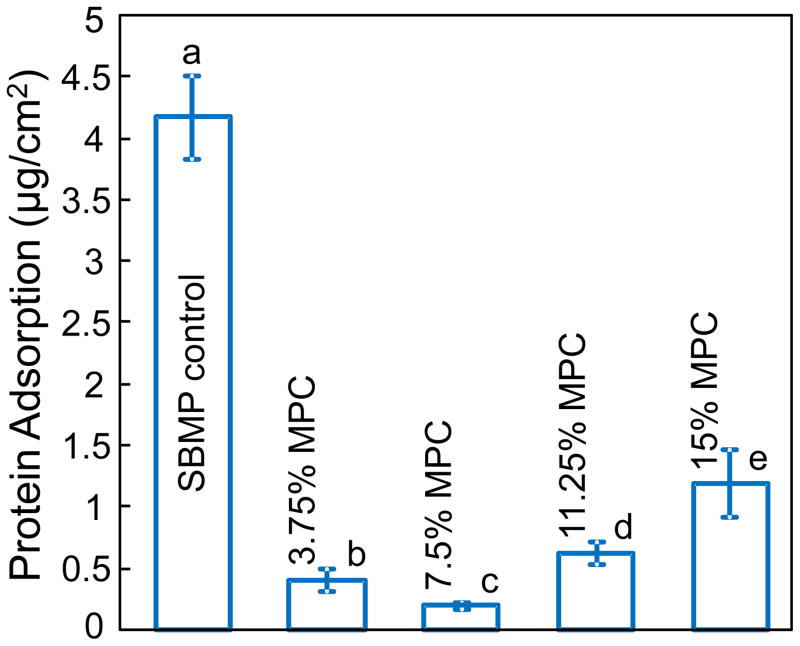
The amounts of protein adsorption on resin disks are plotted in Fig. 2 (mean ± sd; n = 6). Incorporation of MPC into primer and adhesive greatly decreased the amount of protein adsorption, reaching a minimum at 7.5% MPC. Further increasing the MPC mass fraction increased the protein adsorption to the resin surface. These results showed that the resin with 7.5% MPC had the lowest amount of protein adsorption, which was nearly 20-fold less than that of SBMP control.
Fig. 2.
The amount of bovine serum albumin (BSA) protein adsorption onto resin surfaces (mean ± sd; n = 6). Incorporation of MPC into primer and adhesive significantly decreased the amount of protein adsorption on resin surfaces. However, the amount of protein adsorption increased at MPC mass fractions ≥ 11.25%. Dissimilar letters indicate values that are significantly different from each other (p < 0.05).
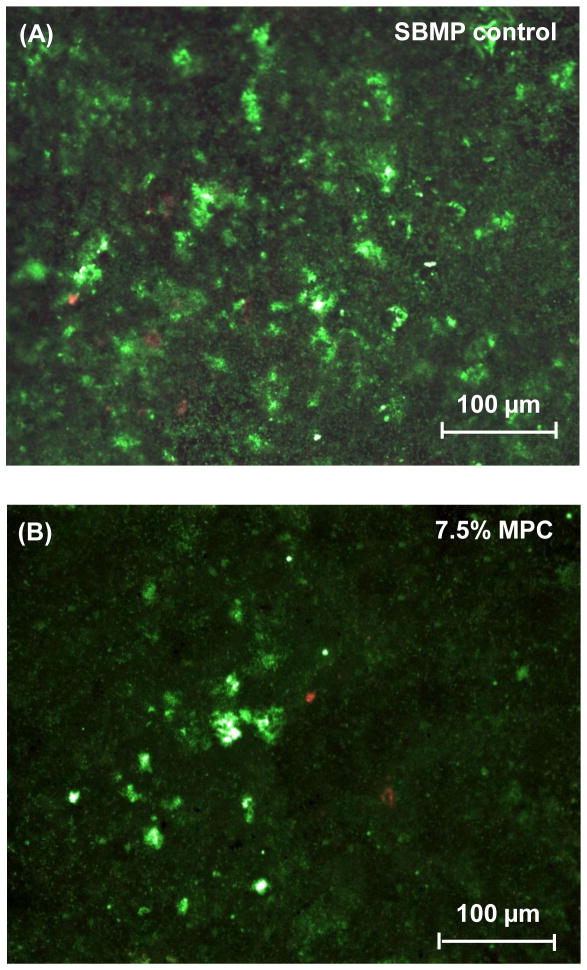
A dental plaque microcosm biofilm model was used with human saliva as inoculum. Fig. 3 shows representative live/dead staining images of 2-day biofilms grown on disks of SBMP control and SBMP containing 7.5% MPC. Live bacteria were stained green, and dead bacteria were stained red. Disks of SBMP control and SBMP containing 7.5% MPC both had primarily live bacteria. However, SBMP control disks were covered with green biofilms. In contrast, SBMP containing 7.5% MPC had much less bacterial adhesion and biofilm coverage on the disks.
Fig. 3.
Representative live/dead staining images of dental plaque microcosm biofilms grown for 2 days on resin disks: (A) SBMP control, (B) SBMP + 7.5% MPC. In (A), biofilms on control disks had primarily live bacteria covering the entire disk. In (B), substantial decreases in bacterial adhesion occurred when MPC was incorporated into primer and adhesive. The live bacteria were stained green, and the dead bacteria were stained red.
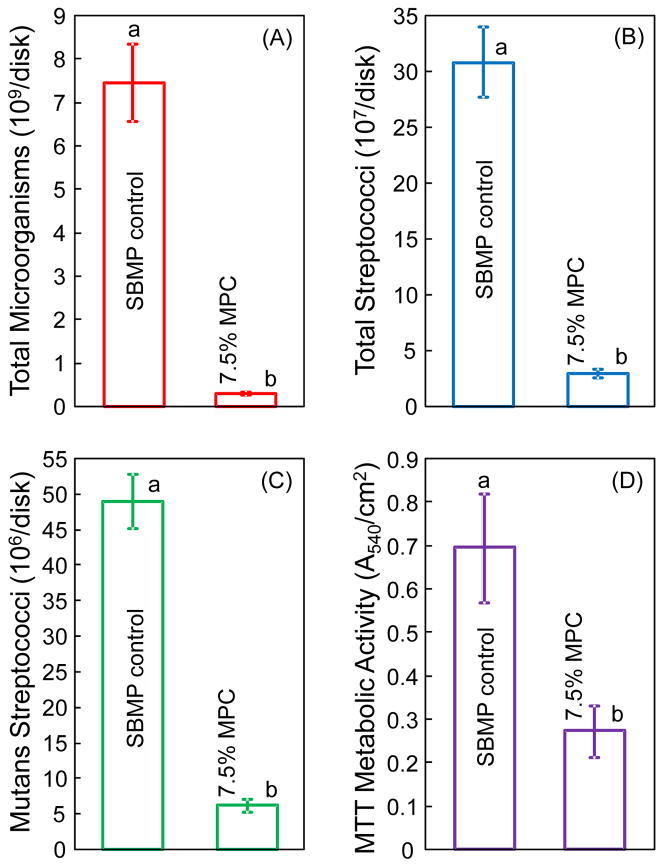
Fig. 4 plots the quantitative 2-day biofilm response on disks of SBMP control and SBMP plus 7.5% MPC: (A) Total microorganisms CFU, (B) total streptococci CFU, (C) mutans streptococci CFU, and (D) metabolic activity (mean ± sd; n = 6). The Kolmogorov-Smirnov test showed that all data had normal distribution. For example, the CFU results had p = 0.968 > 0.05, hence the CFU data had a normal distribution. Incorporation of MPC into primer and adhesive greatly reduced all three CFU counts and the metabolic activity of biofilms, compared to SBMP control (p < 0.05). All three CFU counts of biofilms on resin disks with 7.5% MPC were an order of magnitude lower than those of SBMP control.
Fig. 4.
Colony-forming unit (CFU) counts and metabolic activity of dental plaque microcosm biofilms grown for 2 days on resin disks (mean ± sd; n = 6). (A) Total microorganism CFU, (B) total streptococci CFU, (C) mutans streptococci CFU, and (D) metabolic activity of biofilms. All three CFU counts on the SBMP with 7.5% MPC were an order of magnitude less than that on SBMP control (p < 0.05). Biofilms on SBMP with 7.5% MPC had metabolic activity that was about 40% that of control (p < 0.05).
4. Discussion
The present study represents the first report on the development of protein-repellent dental adhesive resin. This new method successfully reduced protein adsorption on the adhesive resin by an order of magnitude, which in turn reduced oral biofilm CFU by an order of magnitude. The inhibition of bacteria attachment and biofilm growth was achieved without compromising the dentin shear bond strength of the protein-repellent adhesive, compared to the unmodified commercial bonding agent control.
Previous studies indicated that 50–70% of tooth cavity restorations placed by the dentists are replacements of the failed restorations, with secondary caries at the tooth-restoration margins as a primary reason for failure.7,14,19,20 There are usually residual bacteria in the prepared tooth cavity with remnants of carious tissues, which become more common in minimally-invasive management of dental caries.54 In addition, during service in vivo, there are also invading bacteria along the tooth-restoration margins due to bacterial leakage.55,56 Composite restorations are bonded to the tooth structure using bonding agents.21–23 The tooth-restoration interface could degrade due to polymerization shrinkage stresses, cyclic fatigue and wear actions,17,18 hence microgaps could be present at the tooth-restoration margins.17,18 Oral bacteria and biofilms on the margins and those that invade the microgaps would come into contact with the adhesive resin. Cariogenic bacteria in the biofilms can metabolize carbohydrates to organic acids. This in turn can lead to secondary caries at the tooth-restoration margins resulting in restoration failure.
Therefore, it is highly desirable to develop protein-repellent adhesive resins to minimize bacteria attachment and biofilm growth at the weak link, the tooth-restoration margins. Bacterial adhesion is the first step of biofilm formation, and bacterial adhesion follows protein adsorption onto the surfaces.33,34 Therefore, if the resin surface can repel protein adsorption, then it could inhibit bacteria attachment. This could reduce bacterial adhesion and help combat the source of infection. MPC polymer is one of the most common biocompatible and hydrophilic biomedical polymers.39 It was found that MPC could resist nonspecific protein adsorption and bacterial adhesion.40,41 Several medical devices with MPC have been developed and used clinically.42–46 Regarding the protein-repellent mechanism,39,40,57,58 it was suggested that MPC is highly hydrophilic39 and there is an abundance of free water but no bound water in the hydrated MPC polymer.40 In a previous study, the water structure in hydrated MPC polymers was investigated with attention to the free water and it was compared with that in other nonionic amphiphilic polymers.40 The differential scanning calorimetric analysis of these hydrated polymers revealed that the free water fractions in the hydrated MPC polymers were higher than that in other nonionic amphiphilic polymers.40 The presence of bound water would cause protein adsorption.40,57,58 The large amount of free water around the phosphorylcholine group is considered to detach proteins easily, thus repelling protein adsorption.40,58 In the present study, the results of protein adsorption assay confirmed that the incorporation of MPC into primer and adhesive greatly decreased the protein adsorption. However, while the resin with 7.5% MPC had the least protein adsorption, the protein adsorption increased at MPC mass fractions of 11.25% or higher. A possible reason for this is that, when MPC mass fractions were relatively high, the MPC powder could not be completely dissolved in the SBMP primer or adhesive. At MPC mass fractions of 11.25% or higher, MPC could not be completely dissolved in SBMP primer or adhesive, even after the primer or adhesive were stirred for 72 h. This was related to a significant decrease in dentin shear bond strength at 11.25% and 15% MPC (Fig. 1), and this may also have decreased the protein-repellent efficacy of the cured resin. These findings are consistent with previous studies on photo-induced graft polymerization of polymethyl methacrylate47 and polyethylene.59 These previous studies found that although the rate of MPC graft polymerization increased with increasing MPC concentration, the entire polymerization system began to show gelation at higher MPC concentrations, which resulted in a marked decrease in protein-repellent efficiency.47,59 Furthermore, the excess of MPC in the SBMP bonding agents also adversely affected the dentin bond strength. The SBMP bonding agents with high MPC mass fractions (> 11.25%) had significantly lower dentin bond strength. In contrast, the bonding agent with 7.5% MPC had a dentin bond strength similar to that of control (p > 0.1). Taken together, these findings indicate that the use of an optimal MPC mass fraction in the bonding agent is essential to achieve the maximal protein-repellent ability and dentin bond strength. In the present study, incorporation of 7.5% MPC into SBMP primer and adhesive appeared to be optimal in obtaining the highest protein-repellent efficacy, without adversely affecting the dentin bond strength.
Proteins adsorbed onto the resin surface in the oral environment provide a medium for the early attachment of bacteria and microorganisms, thereby initiating the basis for biofilm formation.33,34 Therefore, the fact that the MPC-containing adhesive resin can repel proteins (Fig. 2) would suggest that this resin could potentially also inhibit biofilm growth. Indeed, the results in Figs. 3 and 4 confirmed that the incorporation of MPC at mass fraction of 7.5% into primer and adhesive greatly reduced bacteria adhesion, biofilm CFU and metabolic activity, compared to the commercial bonding agent control. These results demonstrate that the novel idea of the development of protein-repellent dental resins is a promising approach in combating biofilms and inhibiting secondary caries.
The durability of the protein-repellent capability is an important issue. MPC polymers have attracted considerable attention as surface-modifiable polymers for several medical devices.42–44,59,60 Currently, there are two MPC coating methods to modify the polymer surfaces. The first is a conventional MPC polymer coating technique.44,47 MPC can undergo conventional radical copolymerization with other methacrylate such as n-butyl methacrylate (BMA) to form poly(MPC-co-BMA).44 The second method involves photo-induced graft polymerization, in which poly-MPC (PMPC) is grafted onto the substrate through covalent bonding.47,58–60 It was shown that the surface modification layer formed by the photo-induced graft polymerization technique was more resistant to mechanical stress and offered sufficient durability for clinical applications.47,60 The MPC surface modification methods may not be applicable to dental resins, for two reasons. First, dental resins are usually directly placed as a paste into the tooth cavity and then photo-polymerized, which is not suitable for surface modification after placement. Second, polishing, chewing and wear processes will remove the surface layer and the MPC surface modification, therefore losing the MPC and the protein-repellant capability over time. Therefore, in the present study, the MPC was mixed into and co-polymerized with the entire volume of the adhesive resin, so that MPC will be present even after polishing and wear to continue to repel proteins. The SBMP primer and adhesive contained HEMA and a copolymer of acrylic/itaconic acids, which could co-polymerize with MPC. After photo-polymerization, PMPC could be immobilized in the entire resin matrix through covalent bonding, which may offer long-term stability and durable resistance to protein adsorption. However, the long-term protein-repellent activity of MPC-containing dental resins requires further investigation.
MPC-containing dental resins are expected to be useful in a wide range of applications, including protein-repellent bonding agents, composites, sealants, and cements. It should be noted that although MPC-containing resins can reduce bacterial adhesion, they have no bacteria-killing capability. Further studies are needed to incorporate both antibacterial agents and MPC into dental resins to possess the double benefits of protein-repellent and antibacterial capabilities to even more effectively inhibit plaque buildup and combat secondary caries.
5. Conclusions
Protein-repellent dental adhesive resin was developed and evaluated for protein adsorption and biofilm growth properties for the first time. Different mass fractions of MPC were incorporated into dentin primer and adhesive. The hypotheses were verified that incorporation of MPC at mass fraction of 7.5% into the primer and adhesive achieved a strong protein-repellent ability, without compromising the dentin bond strength. The protein-repellent primer and adhesive containing MPC greatly reduced the bacterial adhesion, CFU counts, and metabolic activity of dental plaque microcosm biofilms, compared to commercial bonding agent control. Therefore, the novel protein-repellent MPC-containing dental adhesive may be promising to reduce the bacterial adhesion and biofilm formation at the tooth-restoration margins to reduce secondary caries.
Acknowledgments
We thank Dr. Michael D. Weir and Chen Chen for discussions and experimental help. This study was financially supported by the School of Stomatology at the Capital Medical University in China (NZ), NIH R01 DE17974 (HX), and a Seed Grant (HX) from the University of Maryland School of Dentistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–9. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 2.Hu DY, Hong X, Li X. Oral health in China – trends and challenges. International Journal of Oral Science. 2011;3:7–12. doi: 10.4248/IJOS11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Dental Association (ADA) The 1999 survey of dental services rendered. Chicago, IL: ADA Survey Center; 2002. [Google Scholar]
- 4.Saunders RH, Meyerowitz C. Dental caries in older adults. Dental Clinics of North America. 2005;49:293–308. doi: 10.1016/j.cden.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Curzon MEJ, Preston AJ. Risk groups: nursing bottle caries/caries in the elderly. Caries Research. 2004;38:24–33. doi: 10.1159/000074359. [DOI] [PubMed] [Google Scholar]
- 6.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. Journal of Dental Research. 2008;87:710–9. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferracane JL. Resin composite - State of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Lim BS, Ferracane JL, Sakaguchi RL, Condon JR. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dental Materials. 2002;18:436–44. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 9.Lu H, Stansbury JW, Bowman CN. Impact of curing protocol on conversion and shrinkage stress. Journal of Dental Research. 2005;84:822–6. doi: 10.1177/154405910508400908. [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Ling L, Wang R, Burgess JO. Formation and characterization of a novel fluoride-releasing dental composite. Dental Materials. 2006;22:1014–23. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Lynch CD. Successful posterior composites. London: Quintessence Publishing Co; 2008. [Google Scholar]
- 12.Wei YJ, Silikas N, Zhang ZT, Watts DC. Hygroscopic dimensional changes of self-adhering and new resin-matrix composites during water sorption/desorption cycles. Dental Materials. 2011;27:259–66. doi: 10.1016/j.dental.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Milward PJ, Adusei GO, Lynch CD. Improving some selected properties of dental polyacid-modified composite resins. Dental Materials. 2011;27:997–1002. doi: 10.1016/j.dental.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Primary Dental Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 15.Zalkind MM, Keisar O, Ever-Hadani P, Grinberg R, Sela MN. Accumulation of Streptococcus mutans on light-cured composites and amalgam: an in vitro study. Journal of Esthetic Dentistry. 1998;10:187–90. doi: 10.1111/j.1708-8240.1998.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 16.Beyth N, Domb AJ, Weiss EI. An in vitro quantitative antibacterial analysis of amalgam and composite resins. Journal of Dentistry. 2007;35:201–6. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Loguercio AD, Reis A, Bortoli G, Patzlaft R, Kenshima S, Kenshima S. Influence of adhesive systems on interfacial dentin gap formation in vitro. Operative Dentistry. 2006;31:431–41. doi: 10.2341/05-53. [DOI] [PubMed] [Google Scholar]
- 18.Awliya WY, El-Sahn AM. Leakage pathway of Class V cavities restored with different flowable resin composite restorations. Operative Dentistry. 2008;33:31–6. doi: 10.2341/07-22. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dental Materials. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dental Materials. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. Journal of Biomedical Materials Research. 2002;62:447–56. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 22.Tay FR, Pashley DH. Water treeing--a potential mechanism for degradation of dentin adhesives. American Journal of Dentistry. 2003;16:6–12. [PubMed] [Google Scholar]
- 23.Spencer P, Ye Q, Park JG, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarda K, Katz JL. Adhesive/dentin interface: The weak link in the composite restoration. Annals of Biomedical Engineering. 2010;38:1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J. State of the art of self-etch adhesives. Dental Materials. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. Journal of Dentistry. 1998;26:267–71. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 27.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–9. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 28.Cheng L, Zhang K, Melo MA, Weir MD, Zhou X, Xu HH. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. Journal of Dental Research. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang K, Melo MA, Cheng L, Weir MD, Bai Y, Xu HH. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dental Materials. 2012;28:842–52. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, Ma S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. Journal of Dentistry. 2009;37:289–96. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Hiraishi N, Yiu CK, King NM, Tay FR. Effect of chlorhexidine incorporation into a self-etching primer on dentine bond strength of a luting cement. Journal of Dentistry. 2010;38:496–502. doi: 10.1016/j.jdent.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Lendenmann U, Grogan J, Oppenheim FG. Saliva and dental pellicle – a review. Advances in Dental Research. 2000;14:22–8. doi: 10.1177/08959374000140010301. [DOI] [PubMed] [Google Scholar]
- 33.Kolenbrander PE, London J. Adhere today, here tomorrow: oral bacterial adherence. Journal of Bacteriology. 1993;175:3247–52. doi: 10.1128/jb.175.11.3247-3252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm formation on dental restorative and implant materials. Journal of Dental Research. 2010;89:657–65. doi: 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- 36.Müller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, Imazato S, Ruhl S, Schmalz G. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 2009;30:4921–9. doi: 10.1016/j.biomaterials.2009.05.079. [DOI] [PubMed] [Google Scholar]
- 37.An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. Journal of Biomedical Materials Research. 1998;43:338–48. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 38.Katsikogianni M, Missirlis YF. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria material interactions. European Cells and Materials. 2004;8:37–57. doi: 10.22203/ecm.v008a05. [DOI] [PubMed] [Google Scholar]
- 39.Ishihara K, Ueda T, Nakabayashi N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polymer Journal. 1990;22:355–60. [Google Scholar]
- 40.Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why do phospholipid polymers reduce protein adsorption? Journal of Biomedical Materials Research. 1998;39:323–30. doi: 10.1002/(sici)1097-4636(199802)39:2<323::aid-jbm21>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 41.Ishihara K, Ziats NP, Tierney BP, Nakabayashi N, Anderson JM. Protein adsorption from human plasma is reduced on phospholipid polymers. Journal of Biomedical Materials Research. 1991;25:1397–407. doi: 10.1002/jbm.820251107. [DOI] [PubMed] [Google Scholar]
- 42.Lewis AL. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids and Surfaces B: Biointerfaces. 2000;18:261–75. doi: 10.1016/s0927-7765(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 43.Moro T, Kawaguchi H, Ishihara K, Kyomoto M, Karita T, Ito H. Wear resistance of artificial hip joints with poly(2-methacryloyloxyethyl phosphorylcholine) grafted polyethylene: comparisons with the effect of polyethylene cross-linking and ceramic femoral heads. Biomaterials. 2009;30:2995–3001. doi: 10.1016/j.biomaterials.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Sibarani J, Takai M, Ishihara K. Surface modification on microfluidic devices with 2-methacryloyloxyethyl phosphorylcholine polymers for reducing unfavorable protein adsorption. Colloids and Surfaces B: Biointerfaces. 2007;54:88–93. doi: 10.1016/j.colsurfb.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 45.Kuiper KK, Nordrehaug JE. Early mobilization after protamine reversal of heparin following implantation of phosphorylcholine-coated stents in totally occluded coronary arteries. American Journal of Cardiology. 2000;85:698–702. doi: 10.1016/s0002-9149(99)00843-7. [DOI] [PubMed] [Google Scholar]
- 46.Lewis AL, Tolhurst LA, Stratford PW. Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre-and post-implantation. Biomaterials. 2002;23:1697–706. doi: 10.1016/s0142-9612(01)00297-6. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi N, Iwasa F, Inoue Y, Morisaki H, Ishihara K, Baba K. Evaluation of the durability and antiadhesive action of 2-methacryloyloxyethyl phosphorylcholine grafting on an acrylic resin denture base material. Journal of Prosthetic Dentistry. 2014 doi: 10.1016/j.prosdent.2013.08.020. pii: S0022-3913(13)00351-X. [DOI] [PubMed] [Google Scholar]
- 48.Zhou H, Li F, Weir MD, Xu HH. Dental plaque microcosm response to bonding agents containing quaternary ammonium methacrylates with different chain lengths and charge densities. Journal of Dentistry. 2013;41:1122–31. doi: 10.1016/j.jdent.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antonucci JM, O’Donnell JN, Schumacher GE, Skrtic D. Amorphous calcium phosphate composites and their effect on composite-adhesive-dentin bonding. Journal of Adhesion Science and Technology. 2009;23:1133–47. doi: 10.1163/156856109x432767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McBain AJ. In vitro biofilm models: an overview. Advances in Applied Microbiology. 2009;69:99–132. doi: 10.1016/S0065-2164(09)69004-3. [DOI] [PubMed] [Google Scholar]
- 51.Cheng L, Exterkate RA, Zhou X, Li J, ten Cate JM. Effect of Galla chinensis on growth and metabolism of microcosm biofilms. Caries Research. 2011;45:87–92. doi: 10.1159/000324084. [DOI] [PubMed] [Google Scholar]
- 52.McBain AJ, Sissons C, Ledder RG, Sreenivasan PK, De Vizio W, Gilbert P. Development and characterization of a simple perfused oral microcosm. Journal of Applied Microbiology. 2005;98:624–34. doi: 10.1111/j.1365-2672.2004.02483.x. [DOI] [PubMed] [Google Scholar]
- 53.Lima JP, Sampaio de Melo MA, Borges FM, Teixeira AH, Steiner-Oliveira C, Nobre Dos Santos M, Rodrigues LK, Zanin IC. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. European Journal of Oral Sciences. 2009;117:568–74. doi: 10.1111/j.1600-0722.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 54.Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NH. Minimally invasive management of dental caries: contemporary teaching of posterior resin-based composite placement in U.S. and Canadian dental schools. Journal of the American Dental Association. 2011;142:612–20. doi: 10.14219/jada.archive.2011.0243. [DOI] [PubMed] [Google Scholar]
- 55.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dental Materials. 2007;23:170–6. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 56.Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dental Materials Journal. 2009;28:11–9. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 57.Yamasaki A, Imamura Y, Kurita K, Iwasaki Y, Nakabayashi N, Ishihara K. Surface mobility of polymers having phosphorylcholine groups connected with various bridging units and their protein adsorption-resistance properties. Colloids and Surfaces B: Biointerfaces. 2003;28:53–62. [Google Scholar]
- 58.Goda T, Konno T, Takai M, Ishihara K. Photoinduced phospholipid polymer grafting on Parylene film: Advanced lubrication and antibiofouling properties. Colloids and Surfaces B: Biointerfaces. 2007;54:67–73. doi: 10.1016/j.colsurfb.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Kyomoto M, Moro T, Miyaji F, Hashimoto M, Kawaguchi H, Takatori Y, Nakamura K, Ishihara K. Effects of mobility/immobility of surface modification by 2-methacryloyloxyethyl phosphorylcholine polymer on the durability of polyethylene for artificial joints. Journal of Biomedical Materials Research Part A. 2009;90:362–71. doi: 10.1002/jbm.a.32092. [DOI] [PubMed] [Google Scholar]
- 60.Tateishi T, Kyomoto M, Kakinoki S, Yamaoka T, Ishihara K. Reduced platelets and bacteria adhesion on poly(ether ether ketone) by photoinduced and self-initiated graft polymerization of 2-methacryloyloxyethyl phosphorylcholine. Journal of Biomedical Materials Research Part A. 2014;102:1342–9. doi: 10.1002/jbm.a.34809. [DOI] [PubMed] [Google Scholar]