Abstract
Multiple small injections of streptozotocin produce a delayed, progressive increase in plasma glucose in mice within 5-6 days after the injections, in association with pronounced insulitis and induction of type C viruses within beta cells. Multiple subdiabetogenic doses of streptozotocin in rats and multiple injections of another beta cell toxin, alloxan, in mice did not induce insulitis although hyperglycemia followed the injection of larger quantities of both agents. In mice, the prior injection of 3-O-methyl-D-glucose (3-OMG) or nicotinamide attenuated the diabetic syndrome produced by streptozotocin; however, 3-OMG was more protective. Rabbit antimouse lymphocyte serum, alone, provided partial protection but, when given together with either 3-OMG or nicotinamide, effectively prevented the streptozotocin-induced diabetic syndrome. Cessation of these preventive treatments was followed by the appearance of insulitis and diabetes. These findings suggest that multiple injections of streptozotocin induce, in susceptible hosts, the triad of direct beta cell cytotoxicity, virus induction within beta cells, and cell-mediated autoimmune reaction. These factors, acting separately or in concert, appear to induce a destructive insulitis and severe diabetes. The relative importance of each component and the factors governing host susceptibility remain to be clarified.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craighead J. E., McLane M. F. Diabetes mellitus: induction in mice by encephalomyocarditis virus. Science. 1968 Nov 22;162(3856):913–914. doi: 10.1126/science.162.3856.913. [DOI] [PubMed] [Google Scholar]
- Dulin W. E., Wyse B. M. Studies on the ability of compounds to block the diabetogenic activity of streptozotocin. Diabetes. 1969 Jul;18(7):459–466. doi: 10.2337/diab.18.7.459. [DOI] [PubMed] [Google Scholar]
- From G. L., Craighead J. E., McLane M. F., Steinke J. Virus-induced diabetes in mice. Metabolism. 1968 Dec;17(12):1154–1158. doi: 10.1016/0026-0495(68)90095-4. [DOI] [PubMed] [Google Scholar]
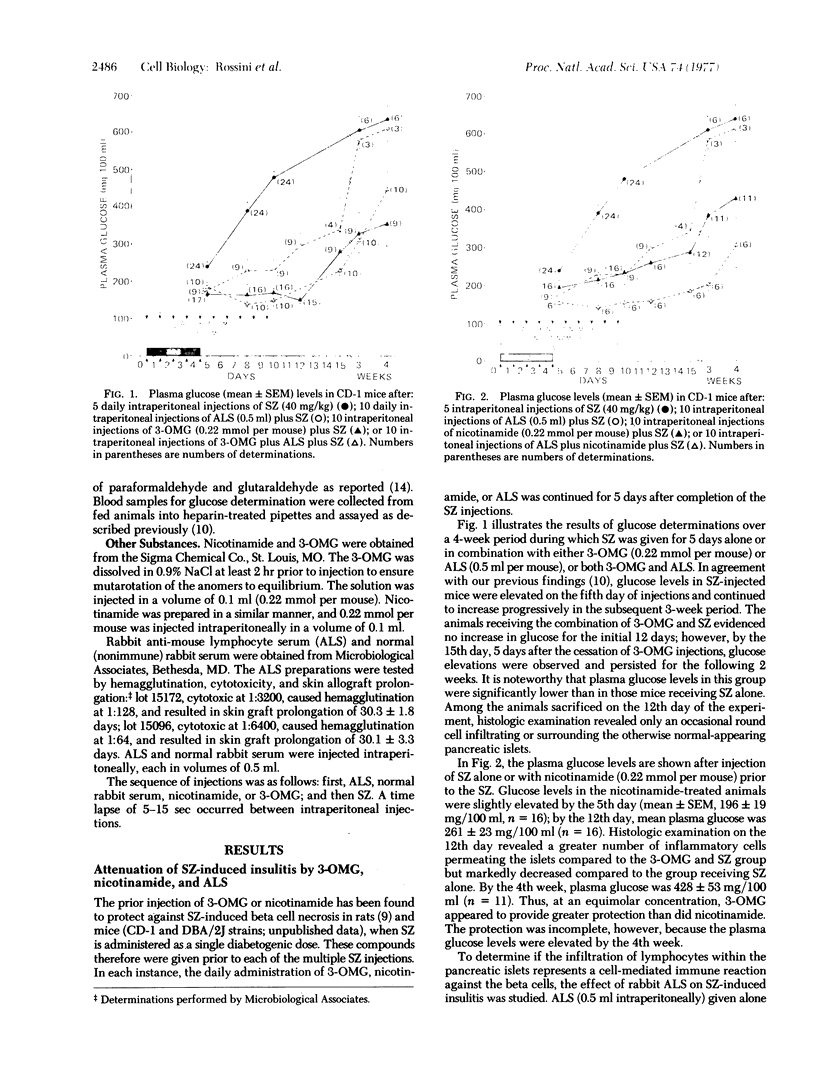
- Ganda O. P., Rossini A. A., Like A. A. Studies on streptozotocin diabetes. Diabetes. 1976 Jul;25(7):595–603. doi: 10.2337/diab.25.7.595. [DOI] [PubMed] [Google Scholar]
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965 Oct;14(10):619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- HERR R. R., EBLE T. E., BERGY M. E., JAHNKE H. K. Isolation and characterization of streptozotocin. Antibiot Annu. 1959;7:236–240. [PubMed] [Google Scholar]
- Junod A., Lambert A. E., Orci L., Pictet R., Gonet A. E., Renold A. E. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967 Oct;126(1):201–205. doi: 10.3181/00379727-126-32401. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Wright P. H. Allergic interstitial pancreatitis in rats injected with guinea pig anti-insulin serum. Diabetes. 1965 Oct;14(10):634–642. doi: 10.2337/diab.14.10.634. [DOI] [PubMed] [Google Scholar]
- Lazarus S. S., Shapiro S. H. Influence of nicotinamide and pyridine nucleotides on streptozotocin and alloxan-induced pancreatic B cell cytotoxicity. Diabetes. 1973 Jul;22(7):499–506. doi: 10.2337/diab.22.7.499. [DOI] [PubMed] [Google Scholar]
- LeCompte P. M., Steinke J., Soeldner J. S., Renold A. E. Changes in the islets of Langerhans in cows injected with heterologous and homologous insulin. Diabetes. 1966 Aug;15(8):586–596. doi: 10.2337/diab.15.8.586. [DOI] [PubMed] [Google Scholar]
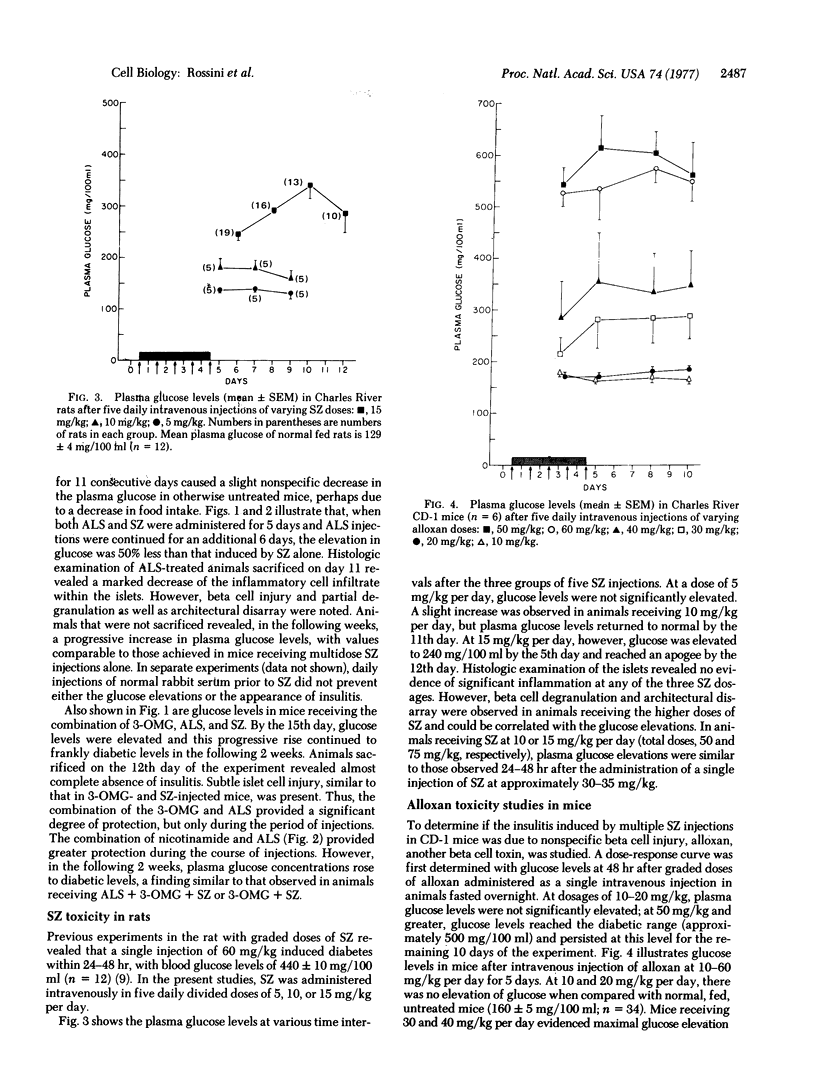
- Like A. A., Orci L. Embryogenesis of the human pancreatic islets: a light and electron microscopic study. Diabetes. 1972;21(2 Suppl):511–534. doi: 10.2337/diab.21.2.s511. [DOI] [PubMed] [Google Scholar]
- Like A. A., Rossini A. A. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976 Jul 30;193(4251):415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Wood M. L., Russell P. S. Effect of adult thymectomy on the recovery from immunological depression induced by anti-lymphocyte serum. Surg Forum. 1965;16:209–211. [PubMed] [Google Scholar]
- Orci L., Amherdt M., Malaisse-Lagae F., Ravazzola M., Malaisse W. J., Perrelet A., Renold A. E. Islet cell membrane alteration by diabetogenic drugs. Lab Invest. 1976 May;34(5):451–454. [PubMed] [Google Scholar]
- RAKIETEN N., RAKIETEN M. L., NADKARNI M. V. Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep. 1963 May;29:91–98. [PubMed] [Google Scholar]
- Rakieten N., Gordon B. S., Cooney D. A., Davis R. D., Schein P. S. Renal tumorigenic action of streptozotocin (NSC-85998) in rats. Cancer Chemother Rep. 1968 Sep;52(5):563–567. [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Schein P., Kahn R., Gorden P., Wells S., Devita V. T. Streptozotocin for malignant insulinomas and carcinoid tumor. Report of eight cases and review of the literature. Arch Intern Med. 1973 Oct;132(4):555–561. [PubMed] [Google Scholar]
- Scheynius A., Täljedal I. B. On the mechanism of glucose protection against alloxan toxicity. Diabetologia. 1971 Aug;7(4):252–255. doi: 10.1007/BF01211877. [DOI] [PubMed] [Google Scholar]
- Stauffacher W., Burr I., Gutzeit A., Beaven D., Veleminsky J., Renold A. E. Streptozotocin diabetes: time course of irreversible B-cell damage; further observations on prevention by nicotinamide. Proc Soc Exp Biol Med. 1970 Jan;133(1):194–200. doi: 10.3181/00379727-133-34439. [DOI] [PubMed] [Google Scholar]
- Toreson W. E., Lee J. C., Grodsky G. M. The histopathology of immune diabetes in the rabbit. Am J Pathol. 1968 May;52(5):1099–1115. [PMC free article] [PubMed] [Google Scholar]
- WAKSMAN B. H., ARBOUYS S., ARNASON B. G. The use of specific "lymphocyte" antisera to inhibit hypersensitive reactions of the "delayed" type. J Exp Med. 1961 Dec 1;114:997–1022. doi: 10.1084/jem.114.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODRUFF M. F., ANDERSON N. F. THE EFFECT OF LYMPHOCYTE DEPLETION BY THORACIC DUCT FISTULA AND ADMINISTRATION OF ANTILYMPHOCYTIC SERUM ON THE SURVIVAL OF SKIN HOMOGRAFTS IN RATS. Ann N Y Acad Sci. 1964 Nov 30;120:119–128. doi: 10.1111/j.1749-6632.1964.tb34710.x. [DOI] [PubMed] [Google Scholar]
- Watkins D., Cooperstein S. J., Lazarow A. Effect of alloxan on islet tissue permeability: protection and reversal by sugars. Am J Physiol. 1973 Mar;224(3):718–722. doi: 10.1152/ajplegacy.1973.224.3.718. [DOI] [PubMed] [Google Scholar]