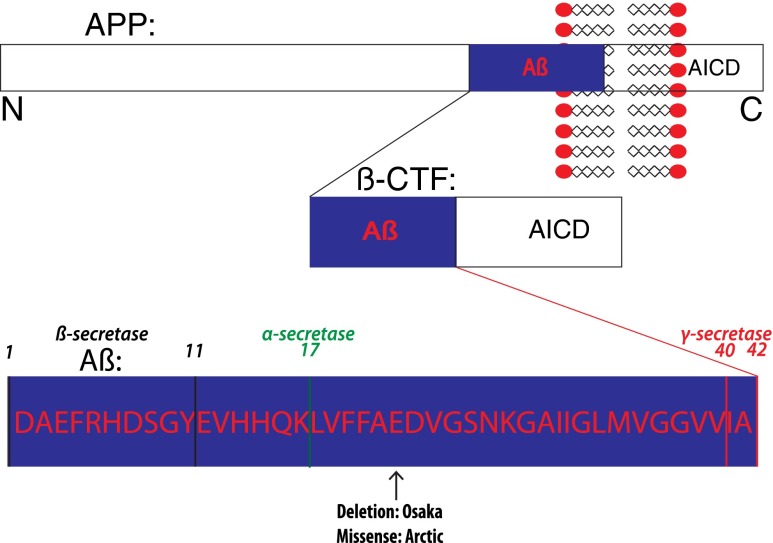
Fig. 1.
A schema of amyloid precursor protein (APP) with its major cleavage sites and familial Alzheimer’s disease (AD) mutations mentioned in the text. Depicted are the amyloidgenic pathway of APP, cleavage sites on the Aβ peptide, and the Arctic and Osaka familial AD mutations. Although other mutations in β-amyloid (Aβ)/APP are not shown, it is important to note that all mutations in APP linked to familial AD are either within Aβ or at the β- or γ-cleavage sites. In the amyloidgenic pathway APP is cleaved by β-secretase to generate C-terminal fragment β (βCTF) and then further cleaved by γ-secretase to generate Aβ. In the nonamyloidgenic pathway, Aβ production is precluded as it is cleaved in the middle by α-secretase. AICD = APP intracellular domain