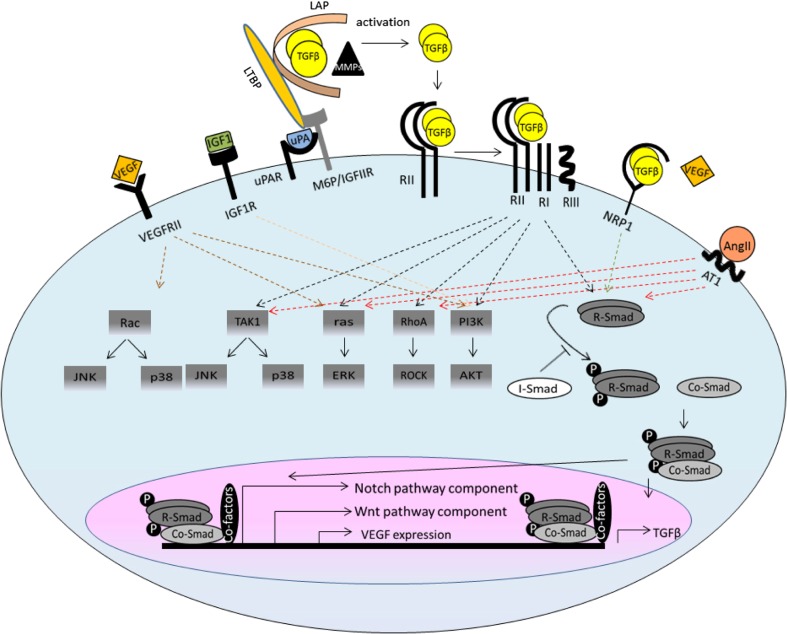
Fig. 1.
Schematic representation of TGFβ signalling and crosstalk with other signalling pathways. TGFβ ligands are synthesised as a large latent complex consisting of TGFβ dimmer covalently associated with a latency-associated peptide (LAP) and a latent TGFβ-binding protein (LTBP). The activation of latent TGFβ requires functional and physical cooperation of M6P/IGFIIR, UPAR, NRP1 and other proteases and MMPs. The released TGFβ dimers bind the type II TGFβ receptor (RII) first, which recruits and transphosphorylates the type I receptors (RI). RI propagates the signal into the cell by phosphorylating TGFβ receptor-regulated SMADs (R-Smads). They form heteromeric complexes with the common SMAD (co-Smad) and translocate to the nucleus. The R-Smads–co-Smad complex formation can be inhibited by inhibitory Smad (I-Smad). Once in the nucleus, the R-SMAD–co-SMAD complex associates with other DNA-binding transcription factors to modulate the expression of target genes. In the non-canonical pathways, the activated transforming growth factor-β (TGFβ) receptor complex transmits a signal through other factors, such as TGFβ-activated kinase 1 (TAK1), p38 mitogen-activated protein kinase (p38 MAPK), RHO, phosphoinositide 3-kinase (PI3K)–AKT, extracellular signal-regulated kinase (ERK), Rho-associated protein kinase (ROCK), or JUN N-terminal kinase (JNK). TGFβ signalling interacts extensively with other pathways, such as the WNT, Notch, AngII, IGF and VEGF pathways, which defines the context-dependent TGFβ signalling