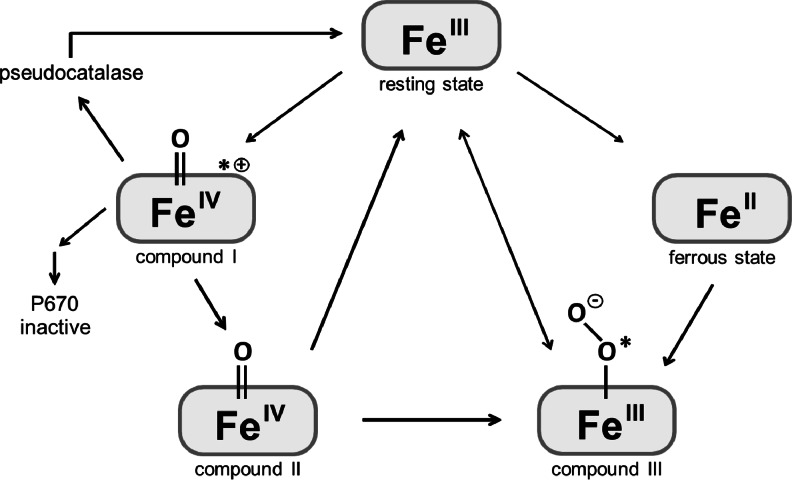
Fig. 3.
Schematic overview of HRP intermediate states. The peroxidative cycle starts with oxidation of the ferric resting state to an oxoferryl species plus a porphyrin-based π cation radical, compound I. Reduction of compound I by elimination of the π cation radical forms compound II which is reduced to return the enzyme to the resting state. Compound III (here shown as superoxide anion-binding ferric species) can be formed from a ferrous species, compound II, or directly from the resting state and slowly decays back to the latter. Upon peroxide excess, compound I can either react back to the resting state via a pseudocatalase activity, react further to compound II, or react to the inactive P670 species