Abstract
A hydroponics experiment was aimed at identifying the lead (Pb) tolerance and phytoremediation potential of Moso bamboo (Phyllostachys pubescens) seedlings grown under different Pb treatments. Experimental results indicated that at the highest Pb concentration (400 μmol/L), the growth of bamboo seedlings was inhibited and Pb concentrations in leaves, stems, and roots reached the maximum of 148.8, 482.2, and 4282.8 mg/kg, respectively. Scanning electron microscopy revealed that the excessive Pb caused decreased stomatal opening, formation of abundant inclusions in roots, and just a few inclusions in stems. The ultrastructural analysis using transmission electron microscopy revealed that the addition of excessive Pb caused abnormally shaped chloroplasts, disappearance of endoplasmic reticulum, shrinkage of nucleus and nucleolus, and loss of thylakoid membranes. Although ultrastructural analysis revealed some internal damage, even the plants exposed to 400 μmol/L Pb survived and no visual Pb toxicity symptoms such as necrosis and chlorosis were observed in these plants. Even at the highest Pb treatment, no significant difference was observed for the dry weight of stem compared with controls. It is suggested that use of Moso bamboo as an experimental material provides a new perspective for remediation of heavy metal contaminated soil owing to its high metal tolerance and greater biomass.
Keywords: Moso bamboo, Pb, Phytoremediation, Scanning electron microscopy, Transmission electron microscopy
1. Introduction
Among heavy metals, lead (Pb) has been acknowledged one of the most abundant metal pollutants in the environment (Patra et al., 2004). Severe Pb contamination may lead to various environmental problems, such as reduced vegetation structure and biodiversity as well as ground water contamination (Ruley et al., 2006). Pb is extremely toxic to plants and humans, leading to brain damage and retardation (Cho-Ruk et al., 2006; Islam et al., 2007).
Phytoremediation can remove heavy metals using plants and offer the benefits of low cost, as well as being an environmentally sustainable technique. To date, about 700 species of plants have been reported to be hyperaccumulators of different contaminants (Xi et al., 2010). Metal hyperaccumulators are found in a large number of plant families, but most are the Brassicaceae family (Verbruggen et al., 2009; Krämer, 2010). Moreover, hyperaccumulators (e.g., Thlaspi sp.) are associated with slow plant growth and low biomass yields (Tandy et al., 2006; Epelde et al., 2008), so there is an urgent need for identification of other plant species having fast growth and greater biomass production. Phytoremediation would be ideal for the recovery of various types of contaminated soil since it is effective and environmentally friendly.
Moso bamboo (Phyllostachys pubescens) grows on more than 3.37 Mha in China, and occupies 70% of the total bamboo in the country (Chen et al., 2009). Compared with other bamboo species, Moso bamboo has some superior attributes in terms of adaptation to environmental conditions, fast growth rate, multipurpose applications, and high ecological values (Wang et al., 2013). Growth rate of Moso bamboo is markedly higher than those of other trees. The average shoot elongation and biomass accumulation rate have been reported to be 17 cm/d and 96 g/d, respectively, and it typically reaches a mature state in less than two months with an average height of 15 m (Xu et al., 2011). In China, Moso bamboo produces biomass of 121.14 t/hm2 (Wu et al., 2002), which is higher than that of the other hyperaccumulator species. Metal hyper-tolerance is usually a constitutive and heritable trait (Macnair, 1993). Sedum alfredii has been found to grow on Pb/Zn mine tailing with shoot Pb concentrations up to 1182 mg/kg. Like S. alfredii, Moso bamboo was also found to grow on the same Pb/Zn mine tailing; therefore, it might have some special tolerance mechanism for these extraordinarily high concentrations of Pb (He et al., 2001; Zhang et al., 2006). However, no research has been conducted to study the effect of Pb on growth, tolerance, or metal accumulation in Moso bamboo.
The aim of the present study was to explore the phytoremediation potential of Moso bamboo plants for its application during remediation of Pb-contaminated soils. The specific objectives were (1) to investigate the growth and physiological responses of Moso bamboo seedlings grown under Pb stress, (2) to investigate the uptake and accumulation of Pb in different plant tissues, and (3) to study the effect of Pb on ultrastructural characteristics of Moso bamboo.
2. Materials and methods
2.1. Plant materials and hydroponics culture
The experiment was conducted under greenhouse conditions at Zhejiang A & F University, China, with the geographic coordinates 30°19′ N, 119°35′ E. Seeds of the Moso bamboo were collected from mature plants growing in Guilin, Guangxi Province, China. Healthy seeds of the Moso bamboo were surface-sterilized by water with 2 g/kg KMnO4 for 30 min. After sterilization, seeds were washed using distilled water and sown in a substrate. On the emergence of seedlings, half-strength Yoshida nutrient solution (Shao et al., 2011) was supplied until seedlings with two leaf-pairs were established.
After two weeks, seedlings with the same size were selected and transferred to plastic pots containing 1.2 L of nutrient solution. The composition of the nutrient solution was as follows (in g/L): 45.7 NH4NO3, 20.15 NaH2PO4·2H2O, 35.7 K2SO4, 44.3 CaCl2, 162 MgSO4·7H2O, 3.73 Na2EDTA, 2.78 FeSO4·7H2O, 0.65 MnSO4·H2O, 0.037 (NH4)6MO7O24·4H2O, 0.467 H3BO3, 0.0175 ZnSO4·7H2O, 0.0155 CuSO4·5H2O, 5.95 citric acid (monohydrate) (C6H8O7), and 0.025 L 98% H2SO4. Different Pb treatments were applied as (1) control (CK), (2) 10 μmol/L, (3) 25 μmol/L, (4) 50 μmol/L, (5) 100 μmol/L, (6) 200 μmol/L, and (7) 400 μmol/L Pb, and source of Pb was Pb(NO3)2. Three plants were grown in each pot and each treatment in triplicate.
2.2. Plant harvest and elemental analysis
The plants were harvested after 30 d and the elements were analyzed according to Islam et al. (2007).
2.3. Analysis of root morphological parameters
After careful washing of the roots of the harvested plants with distilled water to remove any contamination, the root length, diameter, surface area, volume, and tip numbers were recorded by an automatic scanning instrument (Epson Expression 1680) equipped with WinRHIZO software from Regent Instruments (Canada). Plant roots were placed in trays containing 7–10 mm depth of water for scanning and analysis. Special care was taken for untangling roots and to minimize overlapping. Three randomly selected plants from each replicate were selected for data collection.
2.4. Ultrastructural study using electron microscopy
After treatment with 0 and 400 μmol/L Pb for 30 d, plants were selected for the scanning electron microscopy (SEM) and transmission electron microscopy (TEM) study. Small sections (1–3 mm in length) from the middle of the 3rd leaf were used for SEM (Philips Model XL30 ESEM) study. Leaf segments were selected for TEM study from the middle of the 3rd leaf from the top, using a JEOL 1200EX at an accelerating voltage of 60.0 kV (Islam et al., 2007).
2.5. Statistical analysis
Statistical analysis was carried out using statistical package SPSS (Version 13.0). All values reported are means of at least three independent replicates. Data were tested at a significance level of P<0.05 by one-way analysis of variance (ANOVA) (least-significant difference (LSD)). Graphical work was carried out using Sigma Plot software v.12.5.
3. Results
3.1. Seedling growth and biomass production
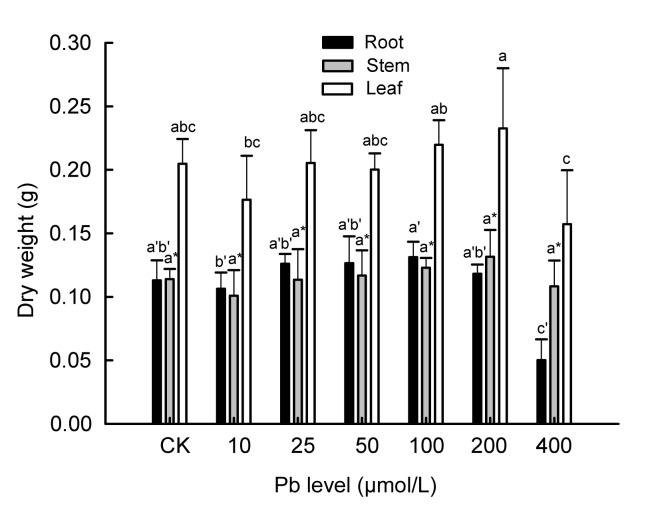
In our hydroponics experiments, Moso bamboo was found to be tolerant to excessive amounts of Pb in nutrient solutions. After treatment with various levels of Pb for 30 d, the plants were healthy and had fully expanded lamina with green coloration at all Pb treatments; no toxic symptoms were observed (Fig. 1). Even the plants exposed to the 400 μmol/L Pb survived and no visual Pb toxicity symptoms such as necrosis and chlorosis were observed in these plants. For the biomass, the dry weight did not decline with the increase of Pb dosage (Fig. 2). At 400 μmol/L Pb treatment, root and leaf dry weights declined significantly (P<0.05); however, even at the highest Pb treatment, no significant difference could be traced for the dry weight of stem compared with CK.
Fig. 1.
Effect of Pb on the growth of Moso bamboo seedlings at the time of harvest
Plants were treated with various levels (0, 10, 25, 50, 100, 200, and 400 μmol/L) of Pb for 30 d
Fig. 2.
Effects of different Pb treatments on dry biomass of Moso bamboo
Error bars are standard deviations (n=3). Different letters indicate significant differences (P<0.05) between the treatment and control (CK)
3.2. Effects of Pb on root morphology
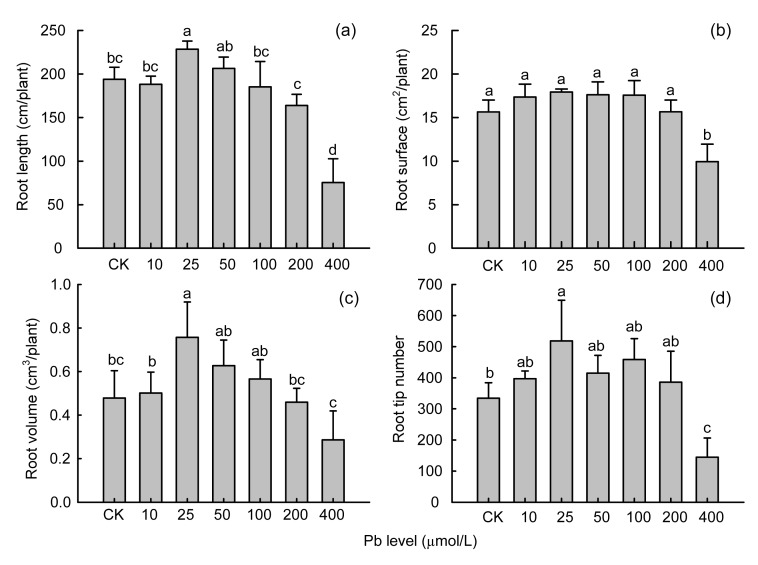
Application of various Pb treatments caused significant changes in root morphological parameters; however, effects were more pronounced at the highest treatment (i.e., 400 μmol/L Pb) (Fig. 3). Fig. 3 shows that after application of Pb up to 200 μmol/L, total root length, root surface, root volume, or number of tips did not decrease much and even values of all four parameters were higher at 25, 50, and 100 μmol/L Pb compared with CK. However, the values of root length and number of tips did show a sharp decline at the 400 μmol/L Pb treatment, where total root length, surface, volume, and number of tips decreased 61%, 37%, 40%, and 57%, respectively, compared with CK.
Fig. 3.
Total root length (a), surface area (b), root volume (c), and number of tips (d) of Moso bamboo showed different responses to different Pb treatments for 30 d
Error bars are standard deviations (n=3). Different letters indicate significant differences (P<0.05) between the treatment and control (CK)
3.3. Absorption, accumulation, and translocation of Pb
After treatment with different Pb levels for 30 d, Pb concentrations increased significantly in the roots and stems in line with increasing Pb concentrations in the culture solution (Table 1). After treatment with different Pb levels, Pb concentrations were highest in roots, followed by stems, and were lowest in leaves. At the 400 μmol/L treatment level, the concentrations of Pb were at the maximum of 4283 and 482 mg/kg in roots and stems, respectively. The transfer factor (TF) and bioaccumulation factor (BCF) tended to be decreased across all the Pb treatments. The TF values ranged from 0.07 (400 μmol/L Pb) to 0.37 (25 μmol/L Pb), while BCF values ranged from 10.97 (400 μmol/L Pb) to 37.64 (10 μmol/L Pb). These high BCF values could be attributed to the high levels of metal accumulated in roots, being several fold higher than the levels in shoots. It is noteworthy that all the TF values were less than 1, whereas all BCF values were more than 10. It is clear from the results that the TF and BCF decreased with increasing Pb concentrations.
Table 1.
Pb concentrations in plant tissues (roots, stems and leaves) of Moso bamboo as affected by Pb treatments
| Pb treatment (μmol/L) | Pb concentration (mg/kg) |
TF | BCF | ||
| Root | Stem | Leaf | |||
| CK | 0.6±0.5b | 0.1±0.7d | 0.2±0.6f | ||
| 10 | 146±30b | 56±14cd | 49±9e | 0.36 | 37.64 |
| 25 | 233±58b | 94±18cd | 84±3d | 0.37 | 24.95 |
| 50 | 331±43b | 141±4cd | 105±8cd | 0.36 | 17.27 |
| 100 | 544±103b | 210±22bc | 127±14bc | 0.29 | 12.82 |
| 200 | 1221±200b | 351±56ab | 165±34a | 0.19 | 11.50 |
| 400 | 4283±994a | 482±166a | 149±17ab | 0.07 | 10.97 |
Values of transfer factor (TF) and bioaccumulation factor (BCF) are shown: TF=C S/C R, BCF=C p/C m, where C S, C R, C p, and C m are Pb concentrations in the shoot, root, plant, and medium, respectively. Different letters in the same column indicate significant differences (P<0.05) among treatments and control (CK)
3.4. Scanning electron microscopy
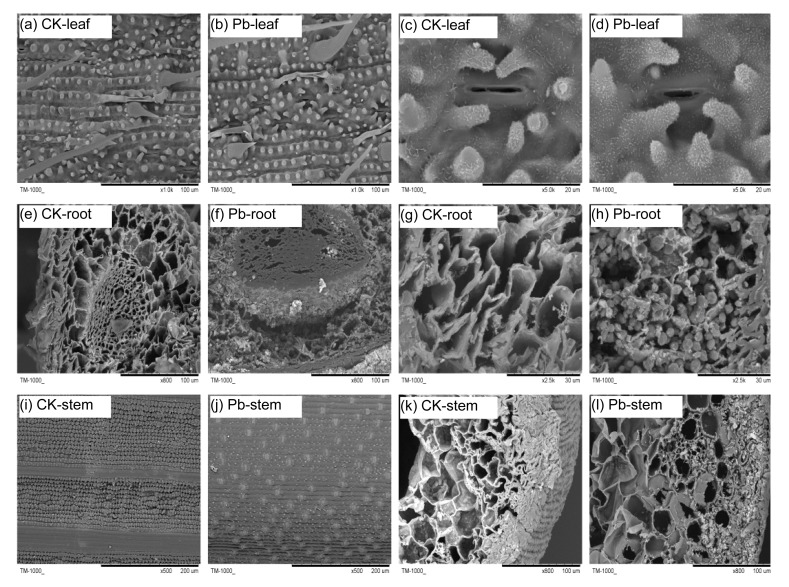
SEM shows that after treating with Pb the surface of the hair became reduced and was smaller slightly than that of CK, and the stomata also diminished (Figs. 4a–4d). Abundant inclusions were observed in roots (Figs. 4e–4h), but very few were observed in stems (Figs. 4i–4l) after treatment with Pb. The protective layer on stem surface disappeared apparently and the numbers of leaf hair reduced significantly as compared with CK.
Fig. 4.
Scanning electron micrographs of cross-sections of Moso bamboo leaf (a–d), root (e–h), and stem (i–l) treated with 0 and 400 μmol/L Pb for 30 d
Control (CK; 0 μmol/L Pb): a, c, e, g, I, and k; 400 μmol/L Pb treated: b, d, f, h, j, and l
3.5. Transmission electron microscopy
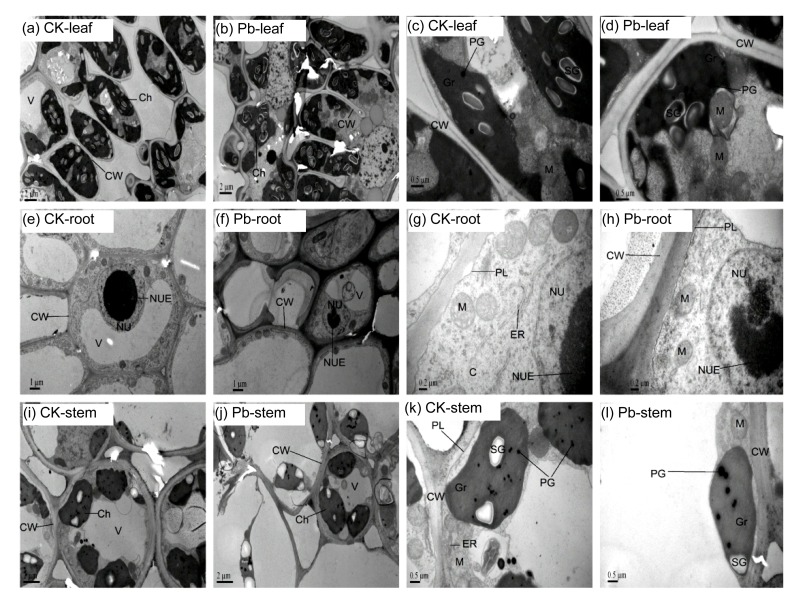
Transmission electron micrographs of the Moso bamboo cells of root, stem, and leaf are shown in Fig. 5. In controls (Figs. 5a, 5c, 5e, 5g, 5i and 5k), the cells of root, stem, and leaf showed the normal performance and no toxicity symptoms were observed. Their cells were filled with cytoplasm with different organelles. Their structure was intact and the cell wall smooth. The oblong chloroplasts with regular arrangement of thylakoid membranes of the grana and starch grains and few plastoglobuli can be observed in both leaf and stem cells. Small vacuoles, a large nucleus and nucleolus, endoplasmic reticulum, and numerous plasmodesmata were visible in root cells of control plants. After treatment with 400 μmol/L Pb, the symptoms of Pb toxicity were observed in these cells. The cell walls in leaf, stem, and root cells were swollen and their structure was distorted (Figs. 5b, 5d, 5f, 5h, 5j and 5l). In leaf cells, abnormally shaped chloroplasts and dissolved thylakoid membranes were observed; however, the shape of mitochondria changed slightly. There were sparse cytoplasm, decreased numbers of mitochondria, large vacuoles, and shrunken nuclei with reduced nucleoli in the meristematic cells of root.
Fig. 5.
Transmission electron micrographs of the leaf (a–d), root (e–h), and stem (i–l)
Ultrathin sections of the Moso bamboo exposed to 0 and 400 μmol/L Pb for 30 d. Control (CK; 0 μmol/L Pb): a, c, e, g, i, and k. 400 μmol/L Pb treated: b, d, f, h, j, and l. Labels: Ch, chloroplast; CW, cell wall; Gr, granum; PG, plastoglobule; M, mitochondrion; PL, plasmalemma; SG, starch grain; NU, nucleus; NUE, nucleolus; V, vacuole; C, cytoplasm; ER, endoplasmic reticulum
4. Discussion
It is known that Pb toxicity is one of the most dangerous environmental pollutants, which inhibits plant growth and development (Cobbett, 2000). Toxicity symptoms include changes in various physiological responses to high concentrations of Pb. These changes include enzyme activity inhibition, mineral nutrition distortion, and membrane permeability imbalance (Sharma and Dubey, 2005).
The superiority of Moso bamboo to maintain its growth potential and to tolerate Pb could, at least partly, be due to selective absorption of essential nutrients from contaminated substrates to maintain appropriate nutrition of their photosynthetic organs (Zaier et al., 2010). Observations on the growth of Moso bamboo and assessment of the root, stem, and leaf revealed that 100 μmol/L of Pb does not affect dry weights, which suggests that Moso bamboo is able to tolerate low doses of Pb. A lesser effect of Pb was observed on stem growth compared with root at high levels of Pb (400 μmol/L), because the first point of contact for this toxic metal is the root (Nada et al., 2007). In a study on willow, when Pb treatments were above the 169 μmol/L, plants showed stunted growth, chlorosis, and serious root biomass reduction. However, Moso bamboo grew healthy and had fully expanded lamina with green coloration when plants were grown at the 200 μmol/L Pb, and no toxic symptoms were observed. In corn seedlings and the non-mined ecotype of Elsholtzia argyi, owing to an increase in the cell wall polysaccharides resulting from Pb exposure, an obvious increase in dry weight of plant organs was reported (Islam et al., 2007). In the present study, the increase in the dry weight of Moso bamboo noted at 200 μmol/L Pb might also be due to the increased synthesis of cell wall polysaccharides in the nutrient solution. It has been reported that when the growth and physiological parameters fall to 50% of CK, plant growth is seriously inhibited (Li et al., 2014). In the present study, the dry weight of Moso bamboo was greater than that of CK in all treatments except for 10 and 400 μmol/L Pb; on the other hand, dry weights only decreased 27% compared with CK even at 400 μmol/L Pb, revealing that Moso bamboo is more suitable for remediation of highly Pb contaminated soil.
Root systems are especially susceptible to heavy metal stress, so the root parameters of plants can be used as important indices (Fan et al., 2011). The results of the present study showed that better root growth and increased Pb uptake by Moso bamboo are attributed to the maintenance of their root activity under Pb stress. Also our results revealed that roots of the Moso bamboo plants have great potential to tolerate and absorb Pb from the growth medium. It was reported that Cucumis sativus could retain greater amounts of metals in the roots on account of its root morphology (An et al., 2004); the greater surface area of these roots is known to contribute more to the absorption of heavy metals and nutrient (Li et al., 2014). Aboveground biomass is also an important indicator of the phytoremediation potential. Pb concentrations in stem tissues of Moso bamboo were 482.25 mg/kg at the 400 μmol/L Pb. Although the concentration did not reach the standard of hyperaccumulators, considering the greater biomass and the leaves occupying only 4% of all biomass of Moso bamboo (Chen et al., 1998), it is still quite valuable for phytoremediation purposes.
Roots are the first organ to come into contact with Pb, and provide the primary route for the penetration of metal ions (Piechalak et al., 2002). It has been well documented that most species roots always accumulated much higher Pb than shoots (López-Millán et al., 2009). Enrichment occurs when heavy metal is taken up by a plant, resulting in an accumulation in the plant. BCF values higher than 1 are indicative of potential hyperaccumulator species (Audet and Charest, 2007). All the BCF were more than 10 and these high BCF values could be attributed mainly to the metal accumulated in the roots several-fold higher than that in the shoots. This high retention corroborates the role of roots, minimizes transport to the shoots, and represents a tolerance mechanism important for phytostabilization processes (Pulford and Watson, 2003). Plants exhibiting TF and particularly BCF values greater than 1 are suitable for phytoremediation. Although Moso bamboo does not reach this standard, it could show normal growth and biomass did not decrease at Pb <400 μmol/L. Given that TF reaches 0.29 at 100 μmol/L Pb, and considering the high biomass values and advantages in postharvest disposal of Moso bamboo, it is suggested that it has great potential as excellent phytoremediation material.
Hyperaccumulators, which retain most of the heavy metal taken up from the soil in the root and detoxify them by chelation in the cytoplasm or storing them in vacuoles, rapidly and efficiently translocate these elements to the shoot via the xylem (Rascio and Navari-Izzo, 2011). There were abundant inclusions observed in roots and very few in stems after treatment with Pb. Metal tolerance was often accompanied by a variety of intracellular changes (Hall, 2002; Sinha et al., 2007). Moso bamboo cell wall, chloroplasts, thylakoid membranes, mitochondria, cytoplasm, vacuoles, and nucleus had a variety of changes, which might reflect metal tolerance capacity of these plants.
Pb toxicity is largely dependent upon absorption, transport, and cellular localization of Pb (Singh et al., 1997). The roots of Moso bamboo had the highest Pb concentrations compared with other tissues. In TEM studies, the root of Moso bamboo was found to contain small aggregates deposited in the cell wall fractions. This deposition pattern partly explains why roots of Moso bamboo were not able to transfer Pb to aerial parts very well, which limits the apoplastic transport of Pb (Rudakova et al., 1988). A probable explanation was that in the cell wall, Pb binds to ion exchangeable sites and with further extracellular precipitation as Pb carbonates (Sharma and Dubey, 2005). Short exposure to Pb may lead to poor translocation of Pb from roots to shoots (Dos Santos Utmazian et al., 2007).
To date, no work has been reported regarding the Pb accumulation potential of Moso bamboo plants. It grows abundantly in China, growing round the year with a high rate of primary production (58.7 t dry weight/ha) (Chen et al., 1998), which shows that Moso bamboo might be a good phytoremediation species considering its large biomass compared with other Pb accumulators. The results of these studies open a new perspective for the selection of plant species for phytoremediation purposes.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31300520), the Science and Technology Program of Zhejiang Province (No. 2014C33043), and the Zhejiang Provincial Natural Science Foundation of China (No. LY12C16004)
Compliance with ethics guidelines: Dan LIU, Song LI, Ejazul ISLAM, Jun-ren CHEN, Jia-sen WU, Zheng-qian YE, Dan-li PENG, Wen-bo YAN, and Kou-ping LU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.An YJ, Kim YM, Kwon TI, et al. Combined effect of copper, cadmium, and lead upon Cucumis sativus growth and bioaccumulation. Sci Total Environ. 2004;326(1-3):85–93. doi: 10.1016/j.scitotenv.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Audet P, Charest C. Heavy metal phytoremediation from a meta-analytical perspective. Environ Pollut. 2007;147(1):231–237. doi: 10.1016/j.envpol.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Hong W, Wei L, et al. Study on biomass and productivity of Phyllostachys heterocycala cv. Pubescens forest in the north of Fujian. Sci Silvae Sin. 1998;34(S1):60–64. (in Chinese) [Google Scholar]
- 4.Chen XG, Zhang XQ, Zhang YP, et al. Changes of carbon stocks in bamboo stands in China during 100 years. Forest Ecol Manag. 2009;258(7):1489–1496. doi: 10.1016/j.foreco.2009.06.051. [DOI] [Google Scholar]
- 5.Cho-Ruk K, Kurukote J, Supprung P. Perennial plants in the phytoremediation of lead-contaminated soils. Biotechnology. 2006;5(1):1–4. doi: 10.3923/biotech.2006.1.4. [DOI] [Google Scholar]
- 6.Cobbett CS. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000;123(3):825–832. doi: 10.1104/pp.123.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dos Santos Utmazian MN, Wieshammer G, Vega R, et al. Hydroponic screening for metal resistance and accumulation of cadmium and zinc in twenty clones of willows and poplars. Environ Pollut. 2007;148(1):155–165. doi: 10.1016/j.envpol.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 8.Epelde L, Hernández-Allica J, Becerril JM, et al. Effects of chelates on plants and soil microbial community: comparison of EDTA and EDDS for lead phytoextraction. Sci Total Environ. 2008;401(1-3):21–28. doi: 10.1016/j.scitotenv.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Fan KC, Hsi HC, Chen CW, et al. Cadmium accumulation and tolerance of mahogany (Swietenia macrophylla) seedlings for phytoextraction applications. J Environ Manag. 2011;92(10):2818–2822. doi: 10.1016/j.jenvman.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 10.Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot. 2002;53(366):1–11. doi: 10.1093/jexbot/53.366.1. [DOI] [PubMed] [Google Scholar]
- 11.He B, Yang XE, Ni WZ, et al. Sedum alfredii: a new lead-accumulating ecotype. Acta Bot Sin. 2001;44(11):1365–1370. (in Chinese) [Google Scholar]
- 12.Islam E, Yang XE, Li TQ, et al. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi . J Hazard Mater. 2007;147(3):806–816. doi: 10.1016/j.jhazmat.2007.01.117. [DOI] [PubMed] [Google Scholar]
- 13.Krämer U. Metal hyperaccumulation in plants. Annu Rev Plant Biol. 2010;61(1):517–534. doi: 10.1146/annurev-arplant-042809-112156. [DOI] [PubMed] [Google Scholar]
- 14.Li SL, Wang FP, Ru M, et al. Cadmium tolerance and accumulation of Elsholtzia argyi origining from a zinc/lead mining site—a hydroponics experiment. Int J Phytoremediat. 2014;16(12):1257–1267. doi: 10.1080/15226514.2013.828010. [DOI] [PubMed] [Google Scholar]
- 15.López-Millán AF, Sagardoy R, Solanas M, et al. Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environ Exp Bot. 2009;65(2-3):376–385. doi: 10.1016/j.envexpbot.2008.11.010. [DOI] [Google Scholar]
- 16.Macnair MR. The genetics of metal tolerance in vascular plants. New Phytol. 1993;124(4):541–559. doi: 10.1111/j.1469-8137.1993.tb03846.x. [DOI] [PubMed] [Google Scholar]
- 17.Nada E, Ferjani BA, Ali R, et al. Cadmium-induced growth inhibition and alteration of biochemical parameters in almond seedlings grown in solution culture. Acta Physiol Plant. 2007;29(1):57–62. doi: 10.1007/s11738-006-0009-y. [DOI] [Google Scholar]
- 18.Patra M, Bhowmik N, Bandopadhyay B, et al. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ Exp Bot. 2004;52(3):199–223. doi: 10.1016/j.envexpbot.2004.02.009. [DOI] [Google Scholar]
- 19.Piechalak A, Tomaszewska B, Baralkiewicz D, et al. Accumulation and detoxification of lead ions in legumes. Phytochemistry. 2002;60(2):153–162. doi: 10.1016/S0031-9422(02)00067-5. [DOI] [PubMed] [Google Scholar]
- 20.Pulford I, Watson C. Phytoremediation of heavy metal-contaminated land by trees—a review. Environ Int. 2003;29(4):529–540. doi: 10.1016/S0160-4120(02)00152-6. [DOI] [PubMed] [Google Scholar]
- 21.Rascio N, Navari-Izzo F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011;180(2):169–181. doi: 10.1016/j.plantsci.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Rudakova EV, Karakis KD, Sidorshina ET. The role of plant cell walls in uptake and accumulation of metal ions. Fiziol Biochim Kult Rast. 1988;20(1):3–12. [Google Scholar]
- 23.Ruley AT, Sharma NC, Sahi SV, et al. Effects of lead and chelators on growth, photosynthetic activity and Pb uptake in Sesbania drummondii grown in soil. Environ Pollut. 2006;144(1):11–18. doi: 10.1016/j.envpol.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Shao JF, Gui RY, Ji HB, et al. A preliminary study on establishment of hydroponic culture system for Phyllostachys pubescens seedlings. J Zhejiang A & F Univ. 2011;28(1):86–94. (in Chinese) [Google Scholar]
- 25.Sharma P, Dubey RS. Lead toxicity in plants. Braz J Plant Physiol. 2005;17(1):35–52. doi: 10.1590/S1677-04202005000100004. [DOI] [Google Scholar]
- 26.Singh RP, Tripathi RD, Sinha S, et al. Response of higher plants to lead contaminated environment. Chemosphere. 1997;34(11):2467–2493. doi: 10.1016/S0045-6535(97)00087-8. [DOI] [PubMed] [Google Scholar]
- 27.Sinha S, Gupta AK, Bhatt K. Uptake and translocation of metals in fenugreek grown on soil amended with tannery sludge: involvement of antioxidants. Ecotoxicol Environ Saf. 2007;67(2):267–277. doi: 10.1016/j.ecoenv.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Tandy S, Schulin R, Nowack B. The influence of EDDS on the uptake of heavy metals in hydroponically grown sunflowers. Chemosphere. 2006;62(9):1454–1463. doi: 10.1016/j.chemosphere.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Verbruggen N, Hermans C, Schat H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009;181(4):759–776. doi: 10.1111/j.1469-8137.2008.02748.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang B, Wei WJ, Liu CJ, et al. Biomass and carbon stock in Moso bamboo forests in subtropical China: characteristics and implications. J Trop For Sci. 2013;25(1):137–148. (in Malay) [Google Scholar]
- 31.Wu JS, Yu YW, Zhu Z, et al. Studies on the biomass of different forests in Huzhou city. J Jiangsu Forest Sci Technol. 2002;29(4):22–24. (in Chinese) [Google Scholar]
- 32.Xi XY, Liu MY, Huang Y, et al. Response of flue-cured tobacco plants to different concentration of lead or cadmium. 2010 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE); IEEE; 2010. pp. 1–4. [DOI] [Google Scholar]
- 33.Xu Y, Wong M, Yang J, et al. Dynamics of carbon accumulation during the fast growth period of bamboo plant. Bot Rev. 2011;77(3):287–295. doi: 10.1007/s12229-011-9070-3. [DOI] [Google Scholar]
- 34.Zaier H, Ghnaya T, Lakhdar A, et al. Comparative study of Pb-phytoextraction potential in Sesuvium portulacastrum and Brassica juncea: tolerance and accumulation. J Hazard Mater. 2010;183(1-3):609–615. doi: 10.1016/j.jhazmat.2010.07.068. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Ye ZQ, Li TQ, et al. Studies on soil microbial activity in areas contaminated by tailings from Pb, Zn mine. J Soil Water Conserv. 2006;20(3):136–140. (in Chinese) [Google Scholar]