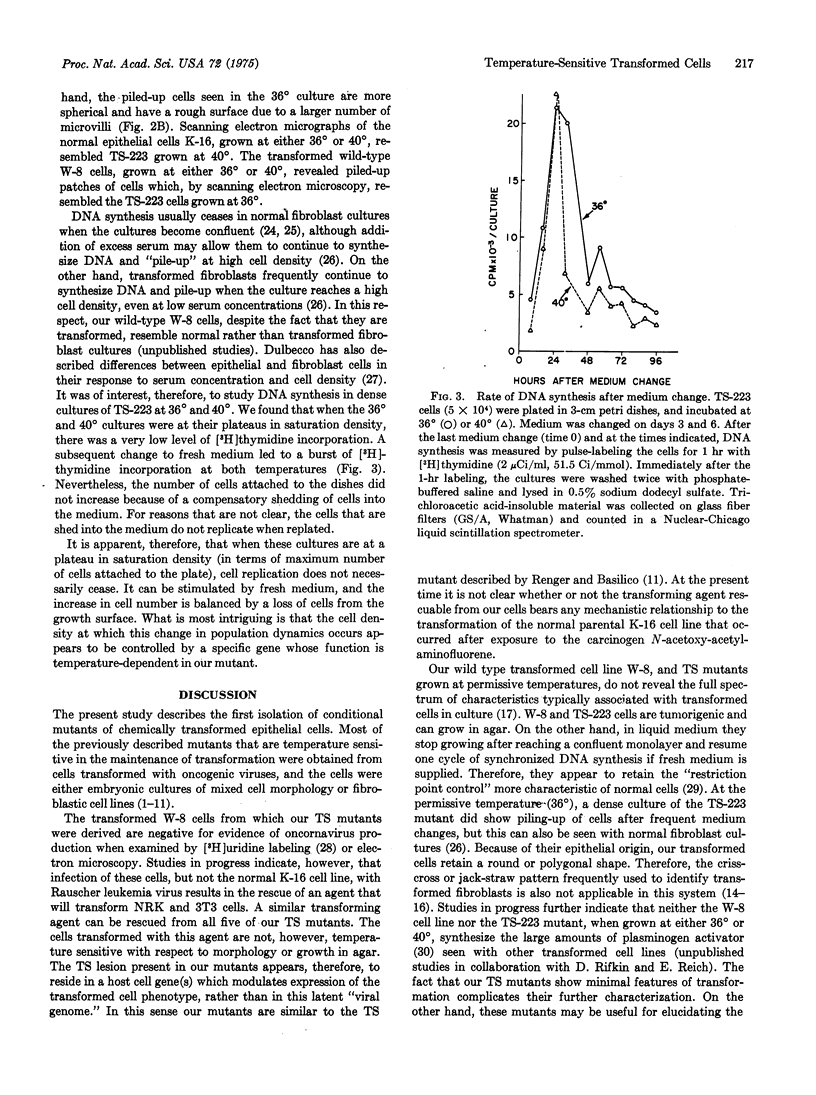
Abstract
The first temperature-sensitive mutants of epithelial cells transformed with chemical carcinogens have been isolated. Like the wild-type transformed parental cells, the mutants readily grow in agar suspension at 36 degrees, but in contrast to the wild type, they do not do so at 40 degrees. Detailed studies of one of these mutants, TS-223, indicate that at high temperature it also has reduced cloning efficiency in monolayer culture and a lower saturation density. Scanning electron microscopy revealed that at 40 degrees confluent cultures of TS-223 consist of a monolayer of generally flat polygonal cells, whereas 36 degrees cultures contain many patches of piled-up cells that are spherical and have rougher surface membranes. All of these cellular changes are reversible with upward or downward temperature shifts. The temperature-sensitive lesion appears to reside in a host cell gene which modulates expression of the transformed cell phenotype. These mutants may provide a useful system for elucidating the minimal biochemical changes required for expression of the transformed phenotype in epithelial cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P. Temperature-dependent transformation of cells infected with a mutant of Bryan Rous sarcoma virus. J Virol. 1972 Aug;10(2):267–276. doi: 10.1128/jvi.10.2.267-276.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biquard J. M., Vigier P. Characteristics of a conditional mutant of Rous sarcoma virus defective in ability to transform cells at high temperature. Virology. 1972 Feb;47(2):444–455. doi: 10.1016/0042-6822(72)90280-2. [DOI] [PubMed] [Google Scholar]
- Chen T. T., Heidelberger C. Quantitative studies on the malignant transformation of mouse prostate cells by carcinogenic hydrocarbons in vitro. Int J Cancer. 1969 Mar 15;4(2):166–178. doi: 10.1002/ijc.2910040207. [DOI] [PubMed] [Google Scholar]
- Chu E. H., Malling H. V. Mammalian cell genetics. II. Chemical induction of specific locus mutations in Chinese hamster cells in vitro. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1306–1312. doi: 10.1073/pnas.61.4.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mayorca G., Greenblatt M., Trauthen T., Soller A., Giordano R. Malignant transformation of BHK21 clone 13 cells in vitro by nitrosamines--a conditional state. Proc Natl Acad Sci U S A. 1973 Jan;70(1):46–49. doi: 10.1073/pnas.70.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Eckhart W. Temperature-dependent properties of cells transformed by a thermosensitive mutant of polyoma virus. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1775–1781. doi: 10.1073/pnas.67.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Elkington J. Conditions limiting multiplication of fibroblastic and epithelial cells in dense cultures. Nature. 1973 Nov 23;246(5430):197–199. doi: 10.1038/246197a0. [DOI] [PubMed] [Google Scholar]
- Friis R. R., Toyoshima K., Vogt P. K. Conditional lethal mutants of avian sarcoma viruses. I. Physiology of ts 75 and ts 149. Virology. 1971 Feb;43(2):375–389. doi: 10.1016/0042-6822(71)90310-2. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakunaga T., Kamahora J. Properties of hamster embryonic cells transformed by 4-nitroquinoline-1-oxide in vitro and their correlations with the malignant properties of the cells. Biken J. 1968 Dec;11(4):313–332. [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells, VII. Induction and isolation of nutritional mutants in Chinese hamster cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1275–1281. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Macpherson I. The characteristics of animal cells transformed in vitro. Adv Cancer Res. 1970;13:169–215. doi: 10.1016/s0065-230x(08)60166-9. [DOI] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger H. C., Basilico C. Mutation causing temperature-sensitive expression of cell transformation by a tumor virus (SV40-3T3 mouse cells-growth control). Proc Natl Acad Sci U S A. 1972 Jan;69(1):109–114. doi: 10.1073/pnas.69.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Stephenson J. R., Aaronson S. A. Isolation of temperature-sensitive mutants of murine sarcoma virus. J Virol. 1972 Oct;10(4):653–657. doi: 10.1128/jvi.10.4.653-657.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers K., Kit S. Temperature-dependent expression of transformation by cold-sensitive mutant of murine sarcoma virus. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2206–2210. doi: 10.1073/pnas.70.8.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Temperature sensitive mutants of an avian sarcoma virus. Virology. 1969 Dec;39(4):930–931. doi: 10.1016/0042-6822(69)90030-0. [DOI] [PubMed] [Google Scholar]
- Unkeless J., Dano K., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Partial purification and characterization of the cell factor, a plasminogen activator. J Biol Chem. 1974 Jul 10;249(13):4295–4305. [PubMed] [Google Scholar]
- Vogel A., Pollack R. Isolation and characterization of revertant cell lines. IV. Direct selection of serum-revertant sublines of SV40-transformed 3T3 mouse cells. J Cell Physiol. 1973 Oct;82(2):189–198. doi: 10.1002/jcp.1040820207. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B., Gebert R., Stadler U. C., Orenstein J. M., Axel R. Type C virus from cell cultures of chemically induced rat hepatomas. Science. 1972 Dec 8;178(4065):1098–1100. doi: 10.1126/science.178.4065.1098. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Elliott J. M., Weisburger J. H. Carcinoma after malignant conversion in vitro of epithelial-like cells from rat liver following exposure to chemical carcinogens. Cancer Res. 1973 Mar;33(3):606–612. [PubMed] [Google Scholar]
- Wyke J. A. Complementation of transforming functions by temperature-sensitive mutants of avian sarcoma virus. Virology. 1973 Jul;54(1):28–36. doi: 10.1016/0042-6822(73)90111-6. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Hirokawa Y. Induction of cell replication. Exp Cell Res. 1968 Oct;52(2):439–444. doi: 10.1016/0014-4827(68)90485-0. [DOI] [PubMed] [Google Scholar]