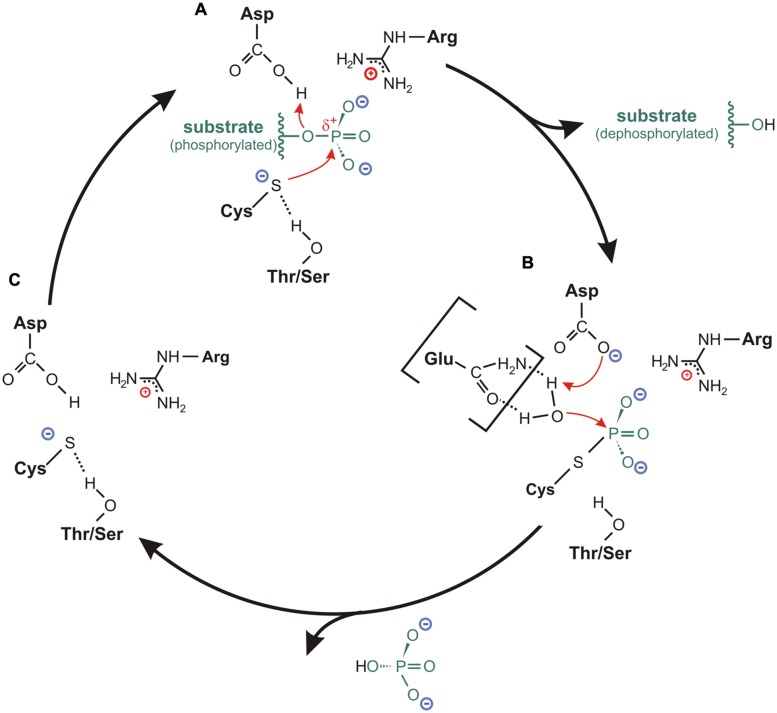
FIGURE 4.
Catalytic cycle for cysteine-based PTPs. (A) In the resting state, the sulfur of the catalytic cysteine is stabilized in an anionic thiolate form. This is mediated by a hydrogen bond between the sulfur and the hydroxyl oxygen of the conserved Thr-/Ser-side chain located in the P-loop. Upon binding of the substrate, where the P-loop arginine is particularly involved in the positioning of the substrate, the thiolate acts as catalytic nucleophile by attacking the phosphorus atom of the substrate. The formation of a cysteinyl-phosphate intermediate is facilitated by a general acid (usually an aspartate from the WPD- or DPYY-loop flanking the active site), which donates a proton to the substrate leaving group. Subsequently, the dephosphorylated substrate dissociates from the substrate binding pocket. (B) The hydrolysis of the cysteinyl-phosphate intermediate is mediated by an activated water molecule. Here, the aspartate or a corresponding residue serves as general base by accepting one proton of the water molecule. In several class I PTPs, the water molecule is activated through hydrogen bonds with one or two polar amino acids in the TI-loop, as it is indicated here for an interaction with a glutamine. After accepting the proton from the general base, the phosphate dissociates from the catalytic cysteine, so that (C) the resting state conformation of the active site is reconfigured.