Abstract
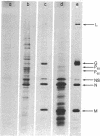
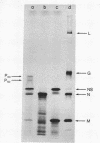
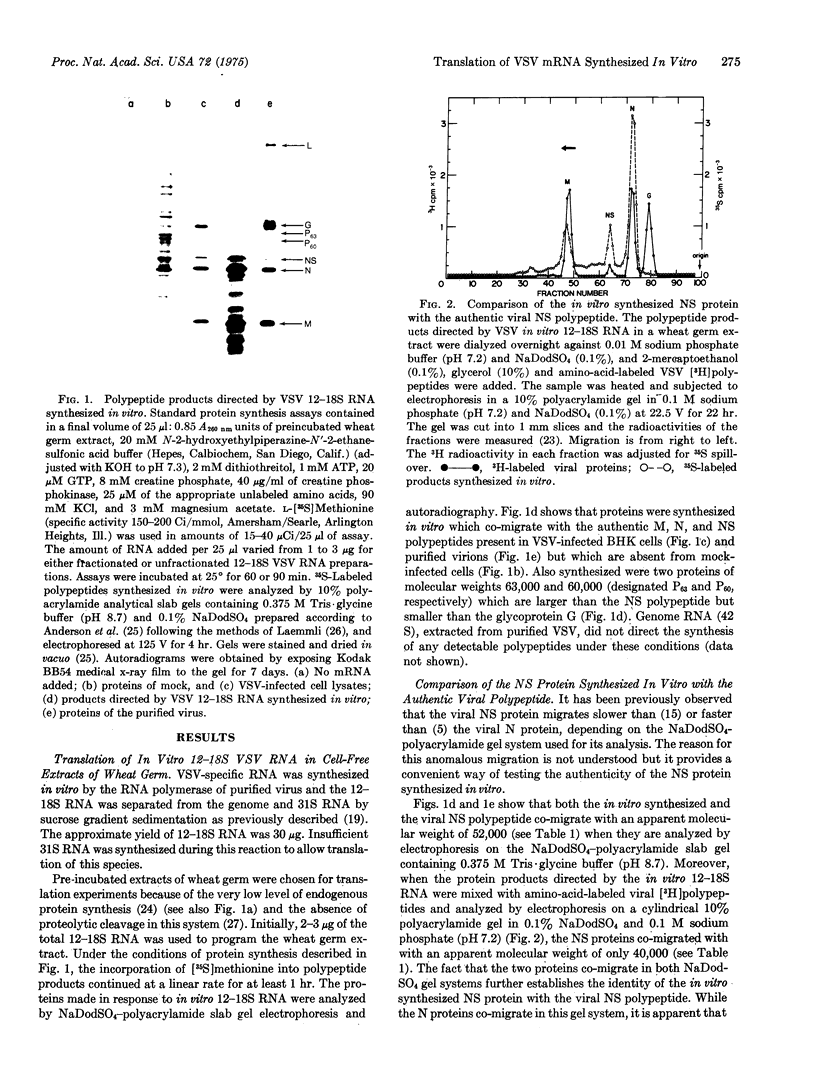
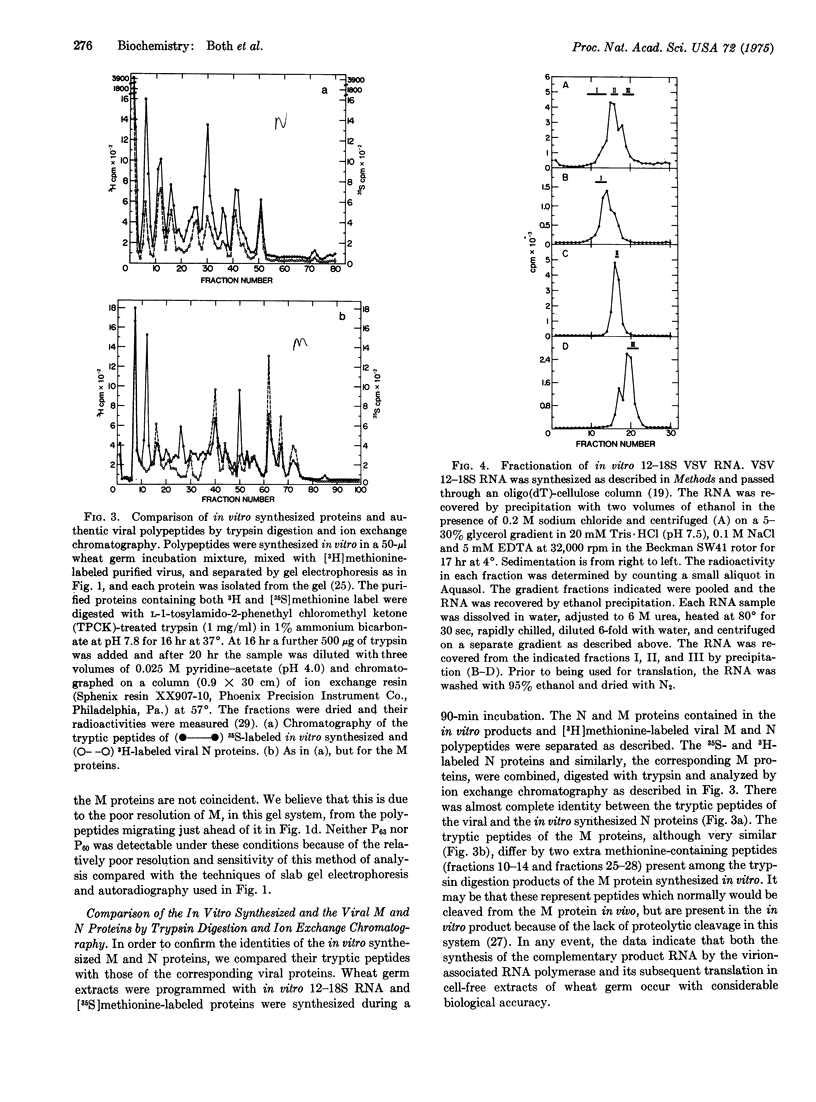
Vesicular stomatitis virus messenger RNA has been transcribed in vitro from the viral genome by the virion-associated RNA polymerase in quantities suitable for translation. Wheat germ cell-free extracts programmed with the isolated in vitro 12-18S RNA fraction synthesize polypeptides similar to the viral N, NS, and M proteins, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and tryptic peptide mapping of the in vitro products and the viral marker polypeptides. In addition, the RNA synthesized in vitro also codes for a protein of molecular weight 63,000 which may be a nonglycosylated form of the viral glycoprotein G. The 12-18S RNA has been partially separated into individual messenger species and these have been identified by the proteins for which they code. There are four monocistronic messenger species in the in vitro 12-18S RNA and the coding capacity of three of these molecules agrees with the estimated molecular weight of the polypeptide assigned to it.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K., Moyer S. A., Rhodes D. P. Studies on the in vitro adenylation of RNA by vesicular stomatitis virus. Virology. 1974 Oct;61(2):547–558. doi: 10.1016/0042-6822(74)90289-x. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Rhodes D. P. In vitro synthesis of RNA that contains polyadenylate by virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3566–3570. doi: 10.1073/pnas.70.12.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H. Complete transcription by the transcriptase of vesicular stomatitis virus. J Virol. 1971 Apr;7(4):486–490. doi: 10.1128/jvi.7.4.486-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F., Martin S. J., Cartwright B., Crick J. The ribonucleic acids of the infective and interfering components of vesicular stomatitis virus. J Gen Virol. 1967 Oct;1(4):479–486. doi: 10.1099/0022-1317-1-4-479. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Huang A. S. Comparison of membrane protein glycopeptides of Sindbis virus and vesicular stomatitis virus. J Virol. 1970 Aug;6(2):176–182. doi: 10.1128/jvi.6.2.176-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F., Hull R. Model for vesicular stomatitis virus. J Virol. 1972 Aug;10(2):256–260. doi: 10.1128/jvi.10.2.256-260.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman M. J., Ehrenfeld E., Summers D. F. In vitro synthesis of proteins by membrane-bound polyribosomes from vesicular stomatitis virus-infected HeLa cells. J Virol. 1974 Sep;14(3):560–571. doi: 10.1128/jvi.14.3.560-571.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Comparative sedimentation coefficients of RNA extracted from plaque-forming and defective particles of vesicular stomatitis virus. J Mol Biol. 1966 Dec 28;22(2):381–384. doi: 10.1016/0022-2836(66)90143-4. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. I. Polyacrylamide gel analysis of viral antigens. J Virol. 1969 Apr;3(4):404–413. doi: 10.1128/jvi.3.4.404-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. II. Immunological comparisons of viral antigens. J Virol. 1970 Jul;6(1):20–27. doi: 10.1128/jvi.6.1.20-27.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrison T., Stampfer M., Baltimore D., Lodish H. F. Translation of vesicular stomatitis messenger RNA by extracts from mammalian and plant cells. J Virol. 1974 Jan;13(1):62–72. doi: 10.1128/jvi.13.1.62-72.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Banerjee A. K. Messenger RNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Cell. 1975 Jan;4(1):37–43. doi: 10.1016/0092-8674(75)90131-2. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Phosphorylation of vesicular stomatitis virus in vivo and in vitro. J Virol. 1974 Feb;13(2):455–465. doi: 10.1128/jvi.13.2.455-465.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Polysomal ribonucleic acid of vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Dec;42(4):958–968. doi: 10.1016/0042-6822(70)90344-2. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- Perrault J., Holland J. J. Absence of transcriptase activity or transcription-inhibiting ability in defective interfering particles of vesicular stomatitis virus. Virology. 1972 Oct;50(1):150–170. [PubMed] [Google Scholar]
- Rhodes D. P., Moyer S. A., Banerjee A. K. In vitro synthesis of methylated messenger RNA by the virion-associated RNA polymerase of vesicular stomatitis virus. Cell. 1974 Dec;3(4):327–333. doi: 10.1016/0092-8674(74)90046-4. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M., Sperling R. The cell-free synthesis and assembly of viral specific polypeptides into TMV particles. Virology. 1974 May;59(1):307–313. doi: 10.1016/0042-6822(74)90227-x. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Howatson A. F. Replication of vesicular stomatitis virus. II. Separation and characterization of virus-specific RNA species. Virology. 1972 Sep;49(3):766–783. doi: 10.1016/0042-6822(72)90533-8. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D. Identification of the vesicular stomatitis virus large protein as a unique viral protein. J Virol. 1973 Apr;11(4):520–526. doi: 10.1128/jvi.11.4.520-526.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R., Banerjee A. K., LaFiandra A., Shatkin A. J. Reovirus-specific ribonucleic acid from polysomes of infected L cells. J Virol. 1972 Jan;9(1):61–69. doi: 10.1128/jvi.9.1.61-69.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]