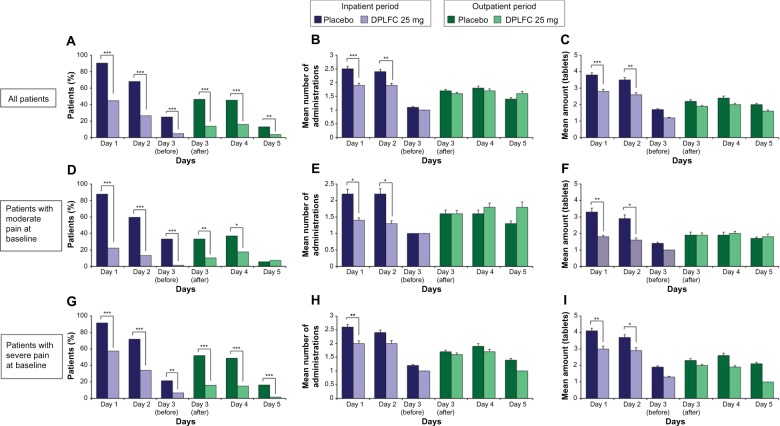
Figure 2.
Summary of opioid RM use.
Notes: Throughout the inpatient (days 1–3) and outpatient (days 3–5) periods, patients received DPLFC every 6 hours and, if needed, opioid RM every 4–6 hours, but at least 1 hour after receiving the study medication. The use of opioid RM (A, D, and G) was analyzed using Cochran–Mantel–Haenszel. The percentage of all patients (A), patients with moderate pain at baseline (D), and patients with severe pain at baseline (G) in the placebo or DPLFC group who used or did not use opioid RM. For patients who used opioid RM, the number of administrations (B, E, and H) and amount of tablets (C, F, and I) were analyzed using one-way analysis of variance. Mean (SEM) number of administrations of opioid RM by all patients (B), patients with moderate pain at baseline (E), and patients with severe pain at baseline (H). Mean (SEM) amount of opioid RM (tablets) for all patients (C), patients with moderate pain at baseline (F), and patients with severe pain at baseline (I). *P<0.05; **P<0.005; ***P<0.0001.
Abbreviations: RM, rescue medication; DPLFC, diclofenac potassium liquid-filled capsule; SEM, standard error of the mean.