OVERVIEW
Prostate cancer is an excellent target for prevention, to reduce both mortality and the burden of overdetection of potential inconsequential disease whose diagnosis increases cost, morbidity, and anxiety. The Prostate Cancer Prevention Trial has demonstrated that finasteride significantly reduces the risk of prostate cancer but only low-grade disease; overall survival is unaffected. In the Selenium and Vitamin E Cancer Prevention Trial (SELECT) clinical trial, selenium had no effect on prostate cancer risk, but alpha tocopherol significantly increased the risk by 17%. The most promising future approaches to prostate cancer prevention will likely focus on nutrition, especially weight control, and through modulation of inflammation.
Prostate cancer is the most common noncutaneous cancer among U.S. men, with 238,590 estimated new cases diagnosed in 2013. Although almost 30,000 men succumbed to this disease in 2013, a great challenge to medical and public health approaches to this disease is that it can be expected that the majority of tumors will never progress to cause symptoms nor will they metastasize and cause death.1 Since the inception of prostate-specific antigen (PSA) testing in the late 1980s, there has been a continued fall in mortality from prostate cancer, but, largely based on the lack of confirmatory evidence from randomized trials of screening as well as the morbidity of treatment of tumors that would likely never have caused morbidity nor mortality, the U.S. Preventive Services Task Force recommended against PSA testing.2 Further demonstrating the challenge with a primary focus on early detection and treatment of prostate cancer is both the very high rate of disease specific survival with active surveillance for localized disease (in which no treatment is provided unless, with time, there is evidence of disease progression) as well as the substantial cost and burden (e.g., repeated biopsies, follow-up clinic visits) of such an approach.3,4
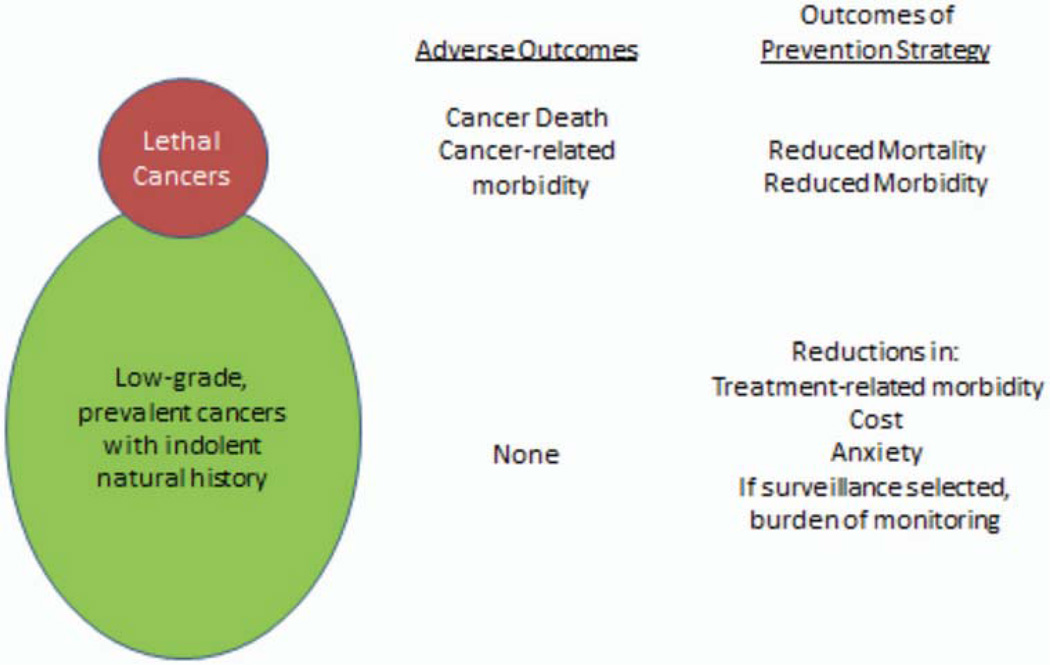
It is the fundamental challenge—that early detection for prostate cancer identifies both potentially lethal cancers as well as a larger fraction of inconsequential tumors and that their futures are difficult to predict, and for this reason, physicians feel obligated, even with a passive, active surveillance approach, to use an intensive, burdensome approach for even the least risky of these tumors—that prevention of prostate cancer is an attractive approach. One consideration, albeit overly simplified, is that, because there are two general forms of prostate cancer—an indolent form as well as an aggressive/lethal form—prevention could have two opportunities. One approach would be those strategies to prevent the aggressive/lethal disease. This approach would have the opportunity to reduce mortality from the disease. A second, somewhat novel approach, would be one that prevents or reduces detection of low-grade/potentially inconsequential cancer. This more novel approach would reduce morbidity from the disease through reducing unnecessary treatments and their adverse consequences and reducing the burden and adverse consequences of an active surveillance strategy. The benefits of either approach are shown in Fig. 1.
FIG 1.
Prostate cancer among aging men is very common but the majority of tumors are low-grade, small, and indolent; a smaller fraction is more aggressive with the potential to cause death. As a result, there are no adverse outcomes of the former, but cancer morbidity and death are adverse outcomes of the latter. Prevention strategies could focus on reducing the clinical diagnosis of either or both of these types of tumors, with the potential benefits listed on the right of the figure.
In this review, we will discuss the outcomes of phase III prostate cancer prevention trials as well as innovative opportunities for future prostate cancer prevention studies.
BASIS FOR COMPLETED PHASE III PROSTATE CANCER PREVENTION TRIALS
The genesis for the two large, phase III prostate cancer prevention trials–the Prostate Cancer Prevention Trial (PCPT) and SELECT (The Selenium and Vitamin E Cancer Prevention Trial)–were two sets of evidence. In the case of the PCPT, the development of the first five alpha reductase inhibitor (finasteride), which mimicked a genetic condition that is protective against prostate cancer and which substantially reduces the androgen stimulus within the prostate (felt to be related to prostate cancer development), prompted the trial development in the early 1990s. The SELECT trial was initiated after evidence from other cancer prevention trials using the two agents (selenium and vitamin E) suggested meaningful reductions in prostate cancer risk. These observations, combined with a body of preclinical data supporting this approach, prompted activation of this study.
PROSTATE CANCER PREVENTION WITH FINASTERIDE: THE PROSTATE CANCER PREVENTION TRIAL
Conceived by the Board of Scientific Counselors of the Division of Cancer Prevention and Control of the National Cancer Institute and overseen by SWOG, the Prostate Cancer Prevention Trial (PCPT) tested the hypothesis whether finasteride, provided to a lower-risk, general population of men over a course of 7 years could reduce the risk of prostate cancer. The population studied were men aged 55 and older with a PSA less than or equal to 3.0 ng/mL. Ultimately, 18,882 men were randomly selected to receive either finasteride, 5 mg daily or placebo. Because finasteride causes an approximate 50% fall in PSA, a fall that changes over time, central PSA monitoring adjusted the annual PSA in the finasteride group to ensure similar number of PSA-prompted biopsy recommendations over the 7 year period of study. Annually, a digital rectal examination (DRE) was performed as well. At the end of the 7-year period, those men without prostate cancer were recommended to undergo prostate biopsy.
The initial report of the study in 2003 was prompted by the independent Data and Safety Monitoring Board who recommended early reporting of the study because the primary endpoint had been met: there was a 24.8% reduction in risk of prostate cancer. The study drug was discontinued, and men were then offered an end-of-study biopsy and the opportunity to enroll in a long-term follow-up study. The initial report was based on data from before the announcement of the outcomes. In that report, although there was a significant reduction in risk of cancer, (803 of the 4,368 patients in the finasteride group [18.4%] and 1,147 of the 4,692 patients in the placebo group [24.4%], a 24.8% reduction in 7-year period prevalence [95% CI, 18.6–30.6 percent; p < 0.001), there was an increased risk of high-grade cancer.1 This prompted the author of the accompanying editorial to recommend against the use of finasteride for chemoprevention of prostate cancer as a result of the concern that although there could be a dramatic reduction in rate of cancer, a higher number of high-grade cancers could increase mortality.6
Subsequent to the original report of the PCPT, a group of studies were conducted to help understand the basis for the prostate cancer paradox: reduced overall risk but higher risk of high-grade cancer. What was subsequently found was that (1) finasteride significantly improved the performance of PSA, leading to improved detection of high-grade cancer7 (for detection of all cancers, the area under the receiver operating characteristic curve for PSA was 0.757 for men receiving finasteride versus 0.681 for men receiving placebo [p < 0.001]; for detection of Gleason grade 7–10 cancers, the area under the receiver operating characteristic curve was 0.838 for finasteride versus 0.781 for placebo [p = 0.003]); (2) finasteride significantly improved the performance of DRE for detection of cancer8 (the sensitivity of DRE for cancer in men receiving finasteride was 21.3% versus 16.7% in men receiving placebo, p = 0.015); (3) finasteride significantly improved the detection of high-grade prostate cancer on biopsy, presumably resulting from improved sampling in the smaller gland that results from drug treatment9 (biopsy of men in the finasteride group identified 69.7% of those men ultimately proven to have high-grade disease as opposed to 50.5% of men in the placebo group [p = 0.01]); and (4) finasteride significantly reduced the risk of detection of high grade prostatic intraepithelial neoplasia (HGPIN; finasteride significantly reduced the overall risk of HGPIN from 570 cases [11.7%] in the placebo group to 420 [9.2%] in the finasteride group [HR = 0.79; 95% CI = 0.70–0.89; p < 0.001]).10 Subsequent studies combining these factors estimated a further reduction in overall risk of prostate cancer and no effect on high-grade disease, as it appeared that the increased detection was likely resulting from improved detection due to these factors caused by finasteride treatment.11
In 2013, almost 20 years after initiation of the PCPT, long-term survival data were examined for the two treatment groups in the PCPT. Using the Social Security Death Index, survival of men treated with finasteride and placebo was studied. In this updated report, of 18,880 eligible study participants, prostate cancer was ultimately found in 1,412 of 9,457 (14.9%) of men in the placebo arm compared with 989 of 9,423 (10.5%) in the finasteride arm, resulting in a 30% relative risk reduction for prostate cancer. Fifteen-year survival rates for the finasteride and placebo arms were similar at 78.0% and 78.2%, respectively. Survival rates for low-grade and high-grade prostate cancers were similar between the two groups as well. This updated study demonstrates that finasteride significantly reduces the risk of prostate cancer with the entirety of the risk reduction being in the low-risk group (the lower component of Fig. 1; among 18,880 eligible men randomly selected in the study, cancer was diagnosed in 989 of 9,423 [10.5%] in the finasteride group compared with 1,412 of 9,457 [14.9% in the placebo group with a relative risk of prostate cancer in the finasteride group of 0.70, 95% CI 0.65–0.76; p < 0.001.)
PROSTATE CANCER PREVENTION WITH VITAMIN E AND SELENIUM: THE SELECT CLINICAL TRIAL
As the PCPT was experiencing rapid accrual of men who were interested in reducing their risk of prostate cancer and with the persistently high rate of prostate cancer detection in the United States, substantial preclinical data was growing suggesting the potential efficacy of alpha tocopherol and selenium for reducing the risk of prostate cancer. Concurrently, secondary analyses of two phase III clinical trials found prostate cancer incidence was reduced with alpha tocopherol and selenium.12,13 This body of evidence led to the activation of the SELECT trial. The study enrolled 35,533 men without prostate cancer and with a normal DRE and a PSA of 4.0 ng/mL or less, randomly selecting them into 4 arms: 200 ug/day l-selenomethionine alone, 400 iu/day of all rac-alpha-tocopheryl acetate, neither, or both. The planned follow-up was a minimum of 7 years and a maximum of 12 years. In September 2008, the independent data and safety monitoring committee met for the second interim analysis and recommended the discontinuation of study supplements as a result of convincing evidence of a lack of benefit from either study agent. In the initial report, the hazard ratios (HRs) for prostate cancer were 1.13 for vitamin E, 1.04 for selenium, and 1.05 for the combination.14 With longer follow-up of study participants in 2011, a subsequent report found that the risk of prostate cancer was significantly increased with vitamin E supplementation alone (HR 1.17, 99% CI [1.004, 1.350], p = 0.008).15 In this report, again, there was no benefit seen with selenium supplementation.
A recently published secondary analysis of SELECT found that the effects of both Se and vitamin E supplementation differed by pre-randomization Se status (as measured by toenail Se concentration).16 Among men with moderate and high Se status at baseline, Se supplementation (Se alone and Se plus vitamin E) increased the risk of high-grade (Gleason 7–10) prostate cancer by 91% (5% CI 20%–205%). Se had no effect on low-grade cancer, regardless of baseline Se status. And among men with low Se status, vitamin E supplementation (vitamin E alone) increased the risk of total cancer by 63% (95% CI 11%–140%) and high-grade cancer by 111% (95% CI 22%–265%). In contrast to the expectation that supplementation of men with low Se status would reduce cancer risk, supplementation of men with moderate and high Se status caused sub-acute Se toxicity that increased cancer risk. And the previously published finding of a 17% increased risk of total cancer among men receiving vitamin E was, more accurately, a much larger increase in risk specifically among men with low Se status at baseline.
The SELECT study has important public health implications. Most importantly, it reiterates findings from previous clinical trials that the use of high-dose micronutrient supplements either have no effect or induce the very cancer they were expected to prevent.17 In the United States, use of highdose micronutrient supplementation is popular but decidedly not benign; it is possible that discontinuing their use could substantially reduce prostate cancer incidence. This study also speaks to the difficulty in translating findings from preclinical data into the realm of actual cancer prevention and the hazard of accepting secondary outcomes of phase III trials, especially in diseases such as prostate cancer, in which biases in disease ascertainment can so strongly affect study results.
FUTURE OPPORTUNITIES FOR PROSTATE CANCER PREVENTION
Obesity, Nutrition, and Vitamins
In 1990, there was a growing consensus that dietary modification and/or dietary supplementation would be powerful tools for prostate cancer prevention. Almost 25 years later, after the completion of multiple randomized trials for primary and secondary prevention along with the continued publication of well-designed epidemiologic studies based on biomarkers of diet (rather than dietary self-report), it has become clear that we were at best naive. Simplistic beliefs about the biologic effects of dietary components (e.g., antioxidants) have been disproved and, most importantly, we are now learning about the significant risks of high-dose supplementation with many commonly used micronutrients (selenium, vitamin E, vitamin D, and long-chain, omega-3 fatty acids).
In contrast to findings about diet/supplements and prostate cancer risk, the previously unclear findings on obesity and prostate cancer are now reasonably consistent and offer a clear, though by no means simple, approach to disease prevention. Before the publication of results from the PCPT, most studies reported that obesity had either no effect on or reduced prostate cancer incidence. This is in contrast to the well-established, though weak, association of obesity with prostate cancer mortality. We now know that obesity affects prostate cancer differentially by grade: it is associated with reduced risk of low-grade and increased risk of high-grade disease. And although the studies on obesity and survival from prostate cancer are inconsistent, there is good evidence that obesity increases the risk of prostate cancer recurrence after “curative” treatment (radiation or prostatectomy). Whether obesity promotes the development of low- to high-grade disease, increases the risk of a high-grade phenotype, or decreases survival by promoting earlier recurrence after treatment is unclear. But based on current evidence, maintaining or achieving a normal weight is, in our opinion, the most scientifically grounded advice we can give a man who is either concerned about getting prostate cancer or who is willing to make lifestyle changes to improve his chances of disease-free survival.
MODULATING INFLAMMATION FOR PREVENTION OF PROSTATE CANCER?
Chronic inflammation is hypothesized to influence prostate cancer development. Prostate biopsies, radical prostatectomy specimens, and tissue resected for treatment of benign prostatic hyperplasia frequently harbor inflammatory cells, especially chronic. This chronic inflammation may serve as an initiator and/or promoter of prostate carcinogenesis much as infections may with respect to liver and stomach cancers, and as inflammatory bowel disease may with respect to colon cancer. Inflammation is an attractive target for intervention. An etiologic link, however, has not been established for prostate cancer.
A complexity to studying prostate cancer in the PSA era is the potential bias that could result from the link between intraprostatic inflammation and a common indication for biopsy, an elevated PSA. In the PCPT, the association between inflammation and prostate cancer risk was uniquely addressable without this bias because of the protocol recommended end-of-study prostate biopsy. We had access to prostate tissue from men who underwent an end-of-study biopsy and did not have prostate cancer detected (control group), and most of the control group did not have an elevated PSA or abnormal PSA.
Intraprostatic inflammation, most of which was chronic, was very common, including in the control group.18 The presence of inflammation was associated with a higher risk of prostate cancer, in particular higher-grade disease. These associations remained after restricting to men in whom intraprostatic inflammation was the least likely to have influenced biopsy recommendation because their PSA was low. Thus, our study supports an etiologic relation between inflammation and prostate cancer, rather than a biased association resulting from the link between intraprostatic inflammation and PSA.
Note that our results differ from two recent studies, one in the Finnish Prostate Cancer Screening Trial,19 and one in REDUCE,20 which found that men with a prior biopsy that was negative for prostate cancer had a reduced prostate cancer risk in the future if inflammation was present in their prior negative biopsy. The PCPT and these study populations are quite different: we did not restrict to men who had a prior negative biopsy. Men with a prior biopsy are likely those who had an elevated PSA, and given that they did not have cancer detected, their PSA elevation was likely a result of intraprostatic inflammation. Thus, men with inflammation on their prior negative biopsy have a lower probability of cancer in the future relative to men without inflammation on their prior negative biopsy.
Although our findings suggest a strategy—intervening on intraprostatic inflammation—for testing for the prevention of prostate cancer, our current study was not prospective in nature. We evaluated the presence of inflammation in the same biopsies that were used to rule in or out the diagnosis of prostate cancer. Our next study takes advantage of the fact that men who did not have a diagnosis of prostate cancer by the end of the PCPT were allowed to enroll in SELECT. We are using the PCPT end-of-study biopsies as the baseline biopsies in SELECT. Unlike the Finnish and REDUCE studies described above, these PCPT-SELECT participants did not have a prior negative biopsy for indication; these biopsies were protocol recommended. Thus, this study will be the first prospective study of intraprostatic inflammation in the etiology of prostate cancer. This work is ongoing.
KEY POINTS.
Opportunities for prostate cancer prevention include both prevention of high-grade, potentially lethal disease (to reduce risk of mortality and morbidity) as well as prevention of low-grade, potentially inconsequential disease (to reduce cost, morbidity of monitoring or treatment, and anxiety).
Finasteride reduces the risk of prostate cancer by 30%; the agent appears to have no to minimal effect on high-grade disease.
Selenium supplementation has no effect on prostate cancer risk.
Vitamin E supplementation significantly increases the risk of prostate cancer by 17%.
Future opportunities for prostate cancer prevention may include dietary or behavioral interventions (typically focused on optimal weight maintenance) and strategies that reduce inflammation.
Acknowledgments
Supported by grants: P30 CA054174, U01 CA86402, CA37429, P01 (CA108964), P30 (CA006973).
Footnotes
Disclosures of Potential Conflicts of Interest
Relationships are considered self-held and compensated unless otherwise noted. Relationships marked “L” indicate leadership positions. Relationships marked “I” are those held by an immediate family member; those marked “B” are held by the author and an immediate family member. Relationships marked “U” are uncompensated.
Employment or Leadership Position: None. Consultant or Advisory Role: None. Stock Ownership: None. Honoraria: None. Research Funding: Ian M. Thompson, NCI. Expert Testimony: None. Other Remuneration: None..
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA. Screening for prostate cancer. US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 3.lvadurai ED, Singhera M, Thomas K, et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur Urol. 2013;64:981–987. doi: 10.1016/j.eururo.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. Clin Oncol. 2010;28:126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 6.Scardino PT. The prevention of prostate cancer—the dilemma continues. N Engl J Med. 2003;349:297–299. doi: 10.1056/NEJMe038109. [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Chi C, Ankerst DP, et al. Effect of finasteride on the sensitivity of PSA for detecting prostate cancer. J Natl Cancer Inst. 2006;98:1128–1133. doi: 10.1093/jnci/djj307. [DOI] [PubMed] [Google Scholar]
- 8.Thompson IM, Tangen CM, Goodman PJ, et al. Finasteride improves the sensitivity of digital rectal examination for prostate cancer detection. J Urol. 2007;177:1749–1752. doi: 10.1016/j.juro.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 9.Lucia MS, Epstein JI, Goodman PJ, et al. Finasteride and high-grade prostate cancer in the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2007;99:1375–1383. doi: 10.1093/jnci/djm117. [DOI] [PubMed] [Google Scholar]
- 10.Thompson IM, Lucia MS, Redman MW, et al. Finasteride decreases the risk of prostatic intraepithelial neoplasia. J Urol. 2007;178:107–109. doi: 10.1016/j.juro.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Redman MW, Tangen CM, Goodman PJ, et al. Finasteride does not increase the risk of high-grade prostate cancer: A bias-adjusted modeling approach. Cancer Prev Res. 2008;1:174–181. doi: 10.1158/1940-6207.CAPR-08-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 13.Clark LC, Combs GF, Jr, Turnbull BW, et al. Nutritional Prevention of Cancer Study Group. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: A randomized controlled trial. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 14.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristal AR, Darke AK, Morris JS, et al. Baseline selenium status and effects of selenium and vitamin E supplementation on prostate cancer risk. J Natl Cancer Inst. doi: 10.1093/jnci/djt456. Epub 2014 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kristal AR, Lippman SM. Nutritional prevention of cancer: New directions for an increasingly complex challenge. J Natl Cancer Inst. 2009;101:363–365. doi: 10.1093/jnci/djp029. [DOI] [PubMed] [Google Scholar]
- 18.Gurel B, Lucia MS, Thompson IM, f, et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the Prostate Cancer Prevention Trial. Accepted Cancer Epidemiol Biomarkers Prev. doi: 10.1158/1055-9965.EPI-13-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yli-Hemminki TH, Laurila M, Auvinen A, et al. Histological inflammation and risk of subsequent prostate cancer among men with initially elevated serum prostate-specific antigen (PSA) concentration in the Finnish prostate cancer screening trial. BJU Int. 2013;112:735–741. doi: 10.1111/bju.12153. [DOI] [PubMed] [Google Scholar]
- 20.Moreira DM, Nickel JC, Gerber L, et al. Baseline prostate inflammation is associated with a reduced risk of prostate cancer in men undergoing repeat prostate biopsy: Results from the REDUCE study. Cancer. 2013. 2014;120:190–196. doi: 10.1002/cncr.28349. [DOI] [PubMed] [Google Scholar]