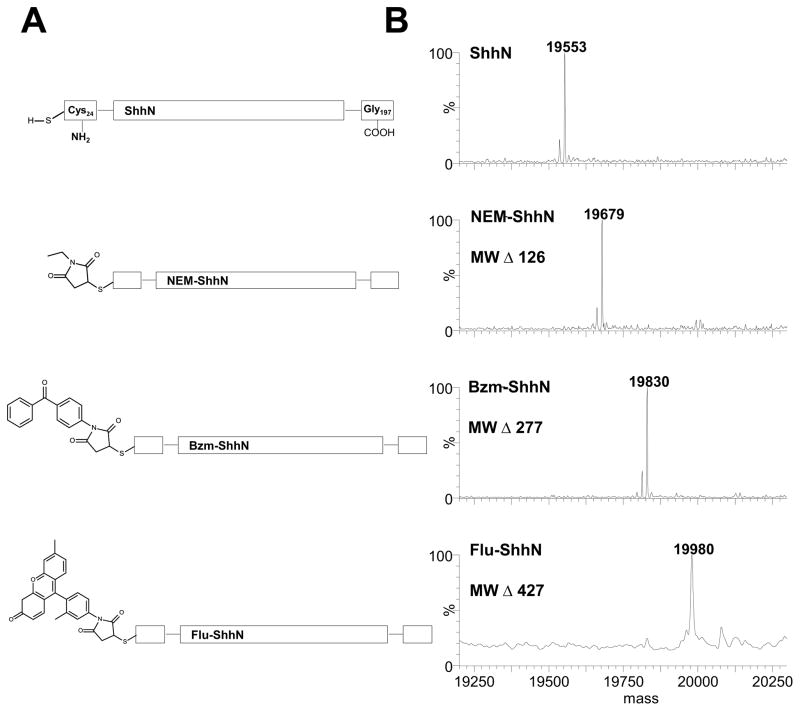
Fig. 1.
Structures and mass spectrometry characterization of modified human Shh proteins. (A) Schematic diagram of Shh proteins with selected modifications of the N-terminal cysteine of Shh. The N-terminal residue in processed human ShhN is cysteine 24 and the C-terminal residue is glycine 197. Unmodified ShhN (ShhN), 5-(N-ethyl-malemidyl) modified ShhN (NEM-ShhN), 4-maleimidobenzophenone modified ShhN (Bzm-ShhN) and fluorescein-maleimide modified ShhN (Flu-ShhN). (B) Whole mass spectra of modified human Shh proteins. ESI-MS spectra of modified Shh proteins with expected change in mass for each modifying group noted.