Figure 3. PLD1 enhances VHL-dependent HIF-1α degradation by accelerating the association between VHL and HIF-1α.
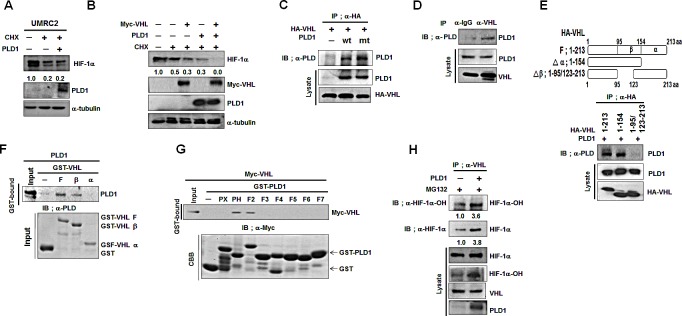
(A) Effect of PLD1 on the stability of HIF-1α in VHL-deficient UMRC cells. Lysates were analyzed by immunoblot and the band intensity was quantified. The levels of HIF-1α to α-tubulin were normalized. Data are representative of three independent experiments. (B) Effect of PLD1 on VHL-mediated endogenous HIF-1α degradation in the presence of CHX. Lysates were analyzed by immunoblot and the band intensity was quantified. The levels of HIF-1α to α-tubulin were normalized. Data are representative of three independent experiments. (C) IP assay of lysatesof HEK293 cells cotransfected with HA-VHL and wtPLD1 or mtPLD1. Data are representative of three independent experiments. (D) IP assay for the interaction of VHL with PLD1. Data are representative of three independent experiments. (E) IP assay for the binding domain mapping of HA-VHL interacting with PLD1. Data are representative of three independent experiments.(F) GST pull-down assay of in vitrotranslated-PLD1 and GST-VHL fragments. Data are representative of threeindependent experiments. (G) GST pull-down assay for the binding domainmapping of PLD1 interacting with VHL. Data are representative of three independent experiments. (H) Effect of PLD1 on the interaction of VHL with hydroxylated HIF-1α in the presence of MG132. The band intensity was quantified and the ratios of hydroxylated HIF-1α to HIF-1α were normalized. Data are representative of three independent experiments.
