Table 2. Reaction with benzyl diethyl phosphate, a less reactive electrophile a .
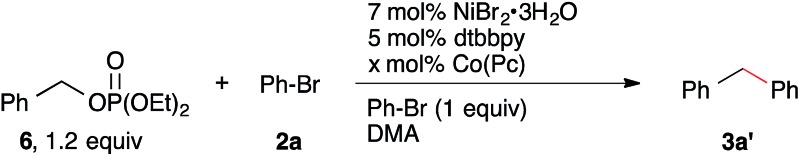
| |||
| Entry | Co(Pc) (mol %) | T (°C) | Yield b |
| 1 | 3 | rt | ND c |
| 2 | 3 | 40 | ND c |
| 3 | 1 | 80 | 3 c , d |
| 4 | 3 | 80 | 36 c , d |
| 5 | 6 | 80 | (70) d |
aReactions run at 0.25 M in DMA using pre-formed 6 for 15–22 h. See ESI.
bYield of 3a′ from GC area% data. Yields in parentheses are isolated yields of purified product.
cSignificant amounts of starting materials remain.
dOnly byproduct observed by GC analysis was small amounts of benzene from hydrodehalogenation of Ph-Br.
