Table 3.
Optimization of palladium-catalyzed DCCP of benzyl phenyl sulfide.
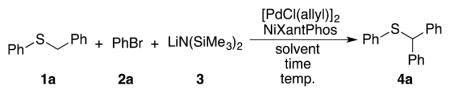
| ||||||
|---|---|---|---|---|---|---|
| entry | 1a:2a:3a | solvent | T (°C) | time (h) | Pd: NiXantPhos (mol %) | 4aa (%) |
| 1 | 2:1:2 | THF | 80 | 12 | 10:20 | 45 |
| 2 | 2:1:3 | THF | 80 | 12 | 10:20 | 0 |
| 3 | 1:2:2 | THF | 80 | 12 | 10:20 | 52 |
| 4 | 1:2:2 | THF | 50 | 12 | 10:20 | 53 |
| 5 | 1:2:2 | THF | R.T. | 12 | 10:20 | 60 |
| 6 | 1:2:2 | THF | R.T. | 6 | 10:20 | 67 |
| 7 | 1:2:2 | THF | R.T. | 1 | 10:20 | 80 |
| 8 | 1:2:2 | THF | R.T. | 0.5 | 10:20 | 86 |
| 9 | 1:2:2 | THF | R.T. | 0.25 | 10:20 | 76 |
| 10 | 1:2:2 | THF | R.T. | 0.5 | 5:10 | 67 |
| 11 | 1:2:2 | THF | R.T. | 0.5 | 5:10 | 64b |
| 12 | 1:2:2 | CPME | R.T. | 0.5 | 10:20 | 0 |
| 13 | 1:2:2 | DME | R.T. | 0.5 | 10:20 | 58 |
| 14 | 1:2:2 | dioxane | R.T. | 0.5 | 10:20 | 0 |
| 15 | 1:2:2 | hexanes | R.T. | 0.5 | 10:20 | 0 |
| 16 | 1:2:2 | toluene | R.T. | 0.5 | 10:20 | 0 |
Yields determined by 1H NMR analysis of crude mixture with CH2Br2 as internal standard.
concentration 0.2 M.
