Table 4.
Substrate scope of aryl bromides in the α-arylation of benzyl phenyl sulfide.
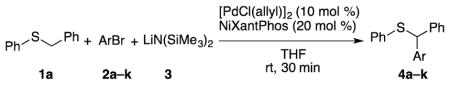
| |||
|---|---|---|---|
| entry | Br–Ar | product | isolated yield (%) |
| 1 | Br–Ph | 4a | 86 |
| 2 |
|
4b | 84a |
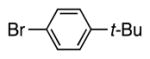
| 3 |
|
4c | 82a |
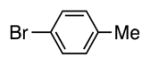
| 4 |
|
4d | 83 |
| 5 |
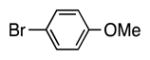
|
4e | 73b |
| 6 |
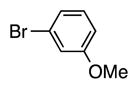
|
4f | 93 |
| 7 |
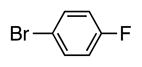
|
4g | 50 |
| 8 |
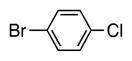
|
4h | 63 |
| 9 |
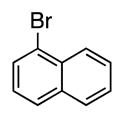
|
4i | 72a |
| 10 |
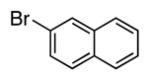
|
4j | 70a |
| 11 |
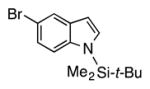
|
4k | 68c |
Slow addition of base for 40 min, 30 min reaction time.
Slow addition of base for 40 min at 10 °C, 15 min reaction time.
4 h reaction time.
