ABSTRACT
We found that the relatively simple microbiota of young infants shifts predictably to a more mature anaerobic microbiota during infancy and the dynamics of this shift are influenced by environmental factors. In this longitudinal study of 75 infants, we demonstrate high interindividual variability within the normal range of birth outcomes, especially in the rate of microbiota progression. Most had acquired a microbiota profile high in Bifidobacterium and Collinsella by 6 months of age, but the time point of this acquisition was later in infants delivered by caesarean section and those born after a shorter duration of gestation. Independently of the delivery mode and gestation duration, infants who acquired a profile high in Bifidobacterium and Collinsella at a later age had lower adiposity at 18 months of age.
IMPORTANCE
This study shows that the acquisition of the early microbiota is strongly influenced by environmental factors such as the delivery mode and duration of gestation, even in healthy neonates. The composition of the early microbiota has been linked with long-lasting effects on health and disease. Here we show that the rate of acquisition of certain microbiota predicts adiposity at 18 months of age and so potentially the risk of later obesity.
INTRODUCTION
The microbes residing in the human gut have a profound influence on our physiology. Variation in the microbiota of the adult human gut has been linked to many outcomes, including a risk of autoimmune disease (1), response to toxins (2), and behavior (3). In particular, the microbial content of the human gut alters the metabolic state (4, 5) and is associated with the development of obesity (4, 6–8) and insulin resistance (9, 10).
At birth, human infants start accumulating intestinal microbiota until a relatively stable state is reached (11). This process is influenced by environmental factors including the mode of delivery, the mode of feeding, the duration of gestation, and the living environment (for reviews, see references 12 and 13). The rate and trajectory of acquisition of a gut microbiota is hypothesized to have a considerable impact on later health outcomes. In particular, disruption of the early microbiota by antibiotic use (14, 15) and a differential trajectory of microbiota acquisition following cesarean section (16, 17) is associated with increased adiposity later in life. Cox et al. (18) recently found that a subtherapeutic dose of penicillin preweaning is sufficient to temporarily alter the mouse gut microbiota and permanently alter the animal’s growth characteristics into adulthood, predisposing it to weight gain and amplifying the effects of a high-fat diet. Crucially, the transient microbial changes caused by penicillin were causative; penicillin-selected microbes were transferred to germ-free hosts and recapitulated the growth promotion phenotype. The precise kind of bacteria encountered by the infant may be important for health outcomes. For example, increased early microbial contact is thought to protect from autoimmune diseases (19), but early colonization with facultative bacterial respiratory pathogens was associated with an increased risk of asthma development (20)
Here we have studied gut microbiota acquisition in 75 infants participating in the Growing Up in Singapore Toward Healthy Outcomes (GUSTO) birth cohort (21). The microbial content of longitudinal fecal samples during the first 6 months of life was profiled by 16S rRNA sequencing, and we investigated the effect of environmental factors, including the delivery mode and duration of gestation, on the trajectories of microbial development, along with the associative relationships with later adiposity.
RESULTS
The infants in this study represented the normal range of birth weights and durations of gestation (Table 1). The microbiotas were simple, with three of the four abundant phyla (Actinobacteria, Proteobacteria, Firmicutes and Bacteroidetes) represented by only one detectable genus and few operational taxonomic units (OTUs); only the phylum Firmicutes showed more complexity at the genus level (see Fig. S1 in the supplemental material). There was substantial interindividual variation in the microbiota composition of infant feces (see Fig. S2 in the supplemental material). Day 3 neonate microbiotas were dominated by Klebsiella OTU2 and Escherichia OTU3, which belong to the family Enterobacteriaceae (of the phylum Proteobacteria), and these levels decreased over time with a commensurate increase in the levels of members of the genus Bifidobacterium (which was the only genus in the phylum Actinobacteria detected) represented by one major OTU. The phylum Firmicutes retained similar abundance levels across time but with changing constituent genera (see Fig. S1). The genus Bacteroides (the only representative of the phylum Bacteroidetes detected) was detected in only a subset of the infants (see Fig. S2).
TABLE 1 .
Characteristics of the subjects included in this studyq
| Parameter | No. of infants | Min | Max | Mean | SD | Median |
|---|---|---|---|---|---|---|
| Maternal age (yr) | 75 | 20 | 40 | 30.85 | 5.09 | 31 |
| Maternal BMIa (kg/m2) | 75 | 17.87 | 40.09 | 27.29 | 5.05 | 26.68 |
| Maternal subscapular skinfold thickness (mm) at 26 wk | 75 | 10.6 | 35 | 21.76 | 5.98 | 21 |
| Gestation duration (wk) | 75 | 35.43 | 41.14 | 38.82 | 1.22 | 39 |
| Infant length (cm) at birth | 69 | 45 | 52.45 | 48.87 | 1.95 | 49 |
| Infant length (cm) at 12 mo | 64 | 69.2 | 82.45 | 75.96 | 3.21 | 76.28 |
| Infant subscapular skinfold thickness (mm) at birth | 72 | 2.74 | 8.3 | 4.99 | 1.19 | 4.9 |
| Infant subscapular skinfold thickness (mm) at 18 mo | 51 | 4.2 | 14 | 6.43 | 1.66 | 6.2 |
| Birth wt (g) | 75 | 2235 | 3980 | 3124.53 | 393.26 | 3110 |
| Wt (g) at 6 mo | 72 | 5882.5 | 9247.5 | 7681.58 | 843.61 | 7650 |
| Wt (g) at 12 mo | 64 | 7195 | 13,110 | 9544.10 | 1123.93 | 9402.5 |
| % of length at 12 mo vs at birthb | 59 | 141.67 | 170.33 | 155.41 | 6.28 | 154.89 |
| % of subscapular skinfold at 18 mo vs at birthc | 50 | 62.65 | 240 | 135.64 | 45.07 | 122.03 |
| % of wt at 6 mo vs at birthd | 72 | 186.55 | 333.12 | 248.57 | 29.78 | 244.88 |
| % of wt at 12 mo vs at 6 moe | 62 | 110.86 | 148.73 | 123.87 | 7.36 | 123.30 |
| Difference between length (cm) at 12 mo and at birthf | 59 | 21.25 | 32.55 | 27.08 | 2.74 | 27 |
| Relative % difference in length at 12 mo and at birthg | 59 | 41.67 | 70.33 | 55.41 | 6.28 | 54.89 |
| Difference in wt (g) at 12 mo and at 6 moh | 62 | 720 | 4055 | 1833.89 | 562.98 | 1718.75 |
| Relative % difference in wt at 12 mo and at 6 moi | 62 | 10.86 | 48.73 | 23.87 | 7.36 | 23.30 |
| Difference in wt (g) at 12 mo and at birthj | 64 | 3977.5 | 9285 | 6406.53 | 1038.50 | 6342.5 |
| % of wt at 12 mo vs at birthk | 64 | 212.04 | 402.06 | 307.72 | 42.76 | 304.65 |
| Relative % difference in wt at 12 mo and at birthl | 64 | 112.04 | 302.06 | 207.72 | 42.76 | 204.65 |
| Difference in wt (g) at 6 mo and at birthm | 72 | 2895 | 6152.5 | 4563.52 | 721.50 | 4496.25 |
| Relative % difference in wt at 6 mo and at birthn | 72 | 86.55 | 233.12 | 148.57 | 29.78 | 144.88 |
| Difference in subscapular skinfold thickness (mm) at 18 mo and at birtho | 50 | −3.1 | 6.9 | 1.4 | 2.02 | 0.98 |
| Relative % difference in subscapular skinfold thickness at 18 mo and at birthp | 50 | −37.35 | 140 | 35.64 | 45.07 | 22.02 |
| Socioeconomic status | 66 | −2.56 | 1.64 | −0.35 | 1.19 | −0.12 |
BMI, body mass index.
(Infant length at 12 months × 100)/length at birth.
(Infant subscapular skinfold thickness at 18 months × 100)/subscapular skinfold thickness at birth.
(Infant weight at 6 months × 100)/weight at birth.
(Infant weight at 12 m × 100)/weight at 6 months.
Infant length at 12 months — length at birth.
[(Infant length at 12 months − length at birth) ×100]/length at birth.
Infant weight at 12 months — weight at 6 months.
[(Infant weight at12 months — weight at 6 months) ×100]/weight at 6 months.
Infant weight at 12 months — birth weight.
(Infant weight at 12 months × 100)/birth weight.
[(Infant weight at 12 months − birth weight) ×100]/birth weight.
Infant weight at 6 months — birth weight.
[(Infant weight at 6 months − birth weight) ×100]/birth weight.
Infant subscapular skinfold thickness at 18 months — subscapular skinfold thickness at birth.
(Infant subscapular skinfold thickness at 18 months — subscapular skinfold thickness at birth) × 100]/subscapular skinfold thickness at birth.
Other characteristics recorded were birth order (25 firstborn, 50 other), gender (39 male, 36 female), antibiotic use in labor (19 yes, 55 no, 1 unknown), delivery mode (57 vaginal, 18 caesarean section), ethnicity (19 Indian, 22 Malay, 34 Chinese), infant feeding at 1 week of age (15 exclusively breastfed, 50 partially breastfed, 10 exclusively formula fed), infant feeding at 3 weeks of age (14 exclusively breastfed, 52 partially breastfed, 9 exclusively formula fed), infant feeding at 3 months of age (13 exclusively breastfed, 18 partially breastfed, 43 exclusively formula fed, 1 unknown), and infant feeding at 6 months of age (7 exclusively breastfed, 13 partially breastfed, 51 exclusive formula fed, 4 unknown).
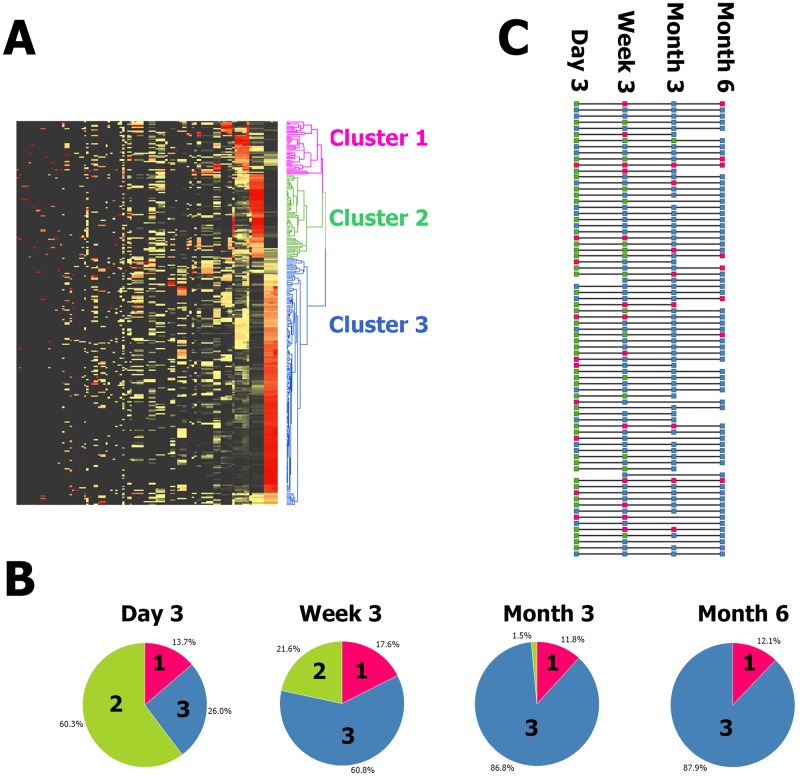
When all of the samples in the study were clustered on the abundance of all of the variable taxa, three deep-rooted clusters were found (Fig. 1A; for an expanded view with taxon labels, see Fig. S3 in the supplemental material). The relationship between the time point and membership of the clusters was highly significant (chi2 P = 1.4−20). Most of the day 3 samples were in cluster 2, which was characterized by high levels of Enterobacteriaceae family members, especially Klebsiella OTU2; in contrast, the majority of the month 6 samples belonged to the biggest cluster, 3, which was characterized by high Bifidobacterium OTU1 and Collinsella levels. Most subjects progressed from cluster 2 to cluster 3 throughout the 6-month time frame. However, at day 3, 19 of the 73 samples were in cluster 3, demonstrating interindividual variability in progression over time (Fig. 1B). Once a sample from an individual was classified in cluster 3, later samples from the same individual tended also to be classified within cluster 3. Finally, there was a minority of samples within cluster 1. Cluster 1 was characterized by very high levels of Firmicutes, in particular, Streptococcus. Although cluster 1 was slightly more frequent in the earlier samples, it appeared across all time points. One subject was stably in cluster 1 across the time points, but others progressed from cluster 2 to cluster 1 to cluster 3 (Fig. 1C).
FIG 1 .
(A) Unsupervised clustering of all samples and all taxa reveals three clusters. Z score-normalized data for all detected taxa are displayed in a heat map with black denoting the minimum value, yellow denoting the average value, and red denoting the maximum value. Each row is a sample, and each column is a taxon. Both taxa and samples are clustered by UPGMA with euclidean distance. A sample dendrogram is shown on the right. The sample dendrogram is pruned at distance 238 (chosen by eye) to reveal three deep-rooted clusters. The clusters are pink, green, and blue and numbered 1, 2, and 3 from the top down. (B) The time point was strongly associated with cluster membership (P = 1.38−20). Pie charts show the proportions of samples at each time point that were classified in each of the three clusters. Sections are colored as in panel A, i.e., magenta for cluster 1, green for cluster 2, and blue for cluster 3, as well as labeled by cluster number. For example, 60% of the day 3 samples were in cluster 2 and no month 6 samples were in cluster 2; meanwhile, 88% of the month 6 samples were in cluster 3 and 26% of the day 3 samples were in cluster 3. (C) Individuals progress from cluster 2 to cluster 3 with some intermediate cluster 1 and much interindividual rate variation. Each horizontal line represents one subject. Each square is colored to reflect the cluster the subject was classified into at each time point. The squares are colored as in panels A and B, i.e., magenta for cluster 1, green for cluster 2, and blue for cluster 3. A progression from cluster 2 (green) to cluster 3 (blue) is typical, but some subjects start at cluster 3 (blue) and some progress through cluster 1 (magenta).
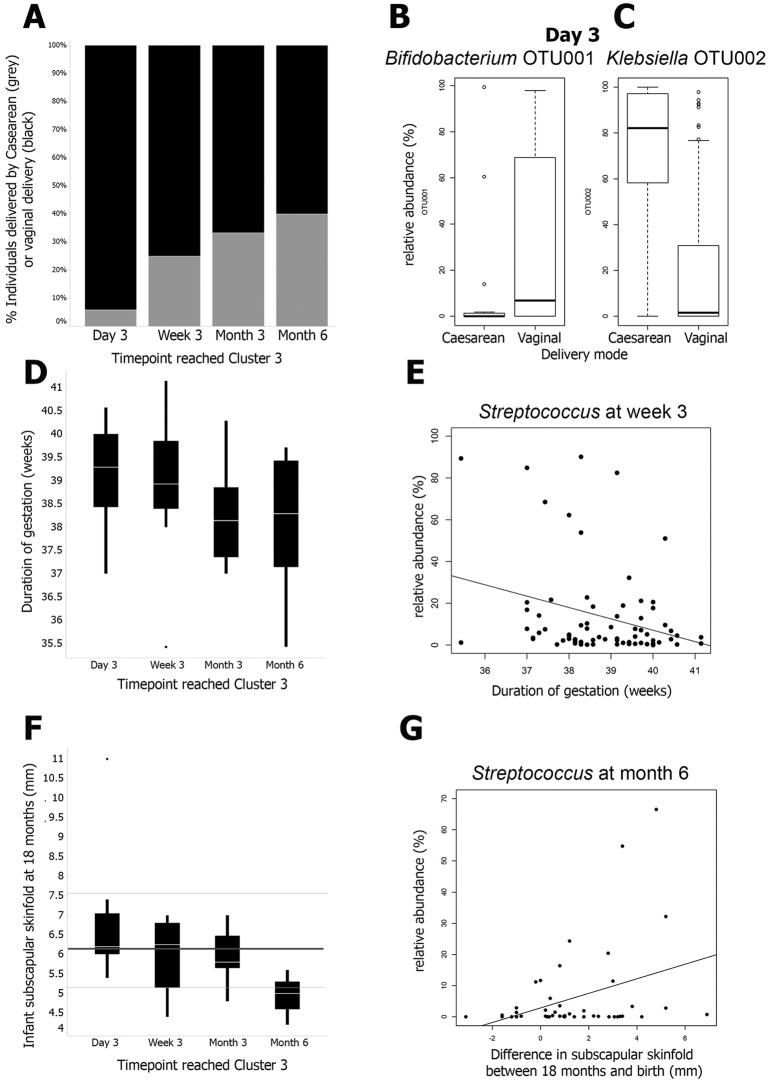
Next, the individual subjects were classified according to the earliest time point at which a sample was classified in cluster 3. Later samples from the same individual also had to be classified in cluster 3. Infants delivered by caesarean section reached (and remained in) cluster 3 later than infants delivered vaginally. Of the 17 individuals who reached a cluster 3 microbiota at day 3, just 1 (6%) was delivered by caesarean section, while 16 had vaginal deliveries; for those reaching cluster 3 at week 3, the month 3 and 6 comparable figures for the proportions delivered by caesarean section were 25% (6 of 24), 33% (5 of 15), and 40% (2 of 5), respectively (Fig. 2A). This is consistent with univariate analysis showing significantly lower levels (P = 0.042) of Bifidobacterium OTU1 detected in babies delivered by caesarean section than in babies delivered vaginally at day 3 (Fig. 2B) and significantly higher levels (P = 5.8E−8) of Klebsiella OTU2 (Fig. 2C).
FIG 2 .
(A) The time point at which a cluster 3 profile is reached is associated with the delivery mode (P = 0.046). On the x axis is the time point at which a cluster 3 profile is reached by each individual, and on the y axis are the proportions of individuals who were born by caesarean delivery (grey) and vaginal delivery (black). (B) Bifidobacterium OTU001 levels are higher in vaginally delivered infants at day 3 than in infants delivered by caesarean section (P = 0.042). The delivery mode is on the x axis, and the relative abundance of Bifidobacterium OTU001 at day 3 is on the y axis. (C) Klebsiella OTU002 levels are lower in vaginally delivered infants at day 3 than in infants delivered by caesarean section (P = 5.8E−6). The delivery mode is on the x axis, and the relative abundance of Klebsiella OTU002 at day 3 is on the y axis. (D) The time point at which a cluster 3 profile is reached is associated with gestational age (P = 0.016). On the x axis is the time point at which a cluster 3 profile is reached by each individual, and on the y axis is the gestational age in weeks. (E) Streptococcus levels are negatively correlated with gestational age at week 3 (P = 0.011). The gestational age is on the x axis, and the relative abundance of the Streptococcus genus at day 3 is on the y axis. (F) The time point at which a cluster 3 profile is reached is associated with infant skinfold thickness at 18 months (P = 0.01). On the x axis is the time point at which a cluster 3 profile is reached by each individual, and on the y axis is infant subscapular skinfold thickness in millimeters at 18 months. For reference, the WHO median subscapular skinfold thickness at 18 months of age of 6.15 mm is denoted by the thick grey line and thin grey lines denote 1 standard deviation from the median (5.15 and 7.5 mm). (G) Month 6 Streptococcus levels are positively correlated with the difference in skinfold thickness (millimeters) between 18 and 0 months (i.e., 18-month skinfold thickness minus neonatal skinfold thickness) (P = 0.018). The difference in skinfold thickness between 18 and 0 months is on the x axis, and the relative abundance of the Streptococcus genus at month 6 is on the y axis.
Babies who reached cluster 3 later tended to be born earlier (Fig. 2D) (P = 0.016 by linear regression). This association survived adjustment for the delivery mode (see Table S1 in the supplemental material) (P = 0.03). The difference in the mean duration of gestation between infants who reached cluster 3 on day 3 and those who did so by month 6 was only 1 week, suggesting that a shorter gestational duration is associated with a lag in microbial acquisition. This observation is consistent with the within-time point analysis showing that babies born after a shorter duration of gestation tend to have higher Streptococcus levels at week 3 (Fig. 2E). There was also an association between reaching cluster 3 later and lower subscapular skinfold thickness at 18 months (Fig. 2F) (P = 0.01 by linear regression); the association survived adjustment for gestational duration and delivery mode (see Table S1) (P = 0.032). The median subscapular skinfold thickness at 18 months in the infants who reached cluster 3 at month 6 was less than 1 standard deviation below the median defined by the WHO (data for both genders combined) (22). Meanwhile, the median subscapular skinfold thickness at 18 months of infants who reached cluster 3 at day 3 and week 3 was similar to the WHO median (22). Interestingly, Streptococcus levels at month 6 were associated with the change in subscapular skinfold thickness between 0 and 18 months (Fig. 2G, P = 0.018 by linear regression); again, the association survived adjustment for gestational duration and delivery mode (see Table S1) (P = 0.02).
DISCUSSION
The infant microbiotas described in our study are simple, with three of the four dominant phyla (Actinobacteria, Proteobacteria, and Bacteroidetes) represented by just one genus and one or two OTUs each. This is largely consistent with other studies (11, 23). The progression of the microbiota over time is also consistent with other findings (11). La Rosa et al. (24) found that the progression of the gut microbiota in the first 40 days of life in premature infants housed in intensive care units from Bacilli to Gammaproteobacteria to Clostridia was remarkably consistent despite relatively restricted exposure to sources of bacteria. Most of our day 3 term infant gut microbiotas were dominated by Gammaproteobacteria (in particular, members of the family Enterobacteriaceae), similar to the day 9 to 17 microbiotas of premature infants. We did not detect the predominant Bacilli phase noted in premature infants, although we did observe that high levels of the Bacilli genus Streptococcus (a marker in our study of clusters 1 and 2) were associated with increased adiposity at 18 months of age. Although Clostridia levels were variable in our infants and increased with time (consistent with reference 24), by month 3, most of our infant microbiotas were dominated by Actinobacteria (in particular, Bifidobacterium).
In this study, the switch between aerobes and anaerobes occurred between birth and month 3 of infancy, in agreement with previous studies (11, 25, 26). However, there was substantial interindividual variation in when the aerobe-anaerobe switch occurred. We observed that gut microbiota of infants in our study could be classified into three distinct clusters (Fig. 1A). Cluster 3 contained ~88% of the month 6 samples and could be said to represent an anaerobic microbiota appropriate for infants in this age range. Twenty-six percent of our infants had reached cluster 3 by day 3. Cluster 3 was characterized by high Bifidobacterium, high Collinsella, low Enterobacteriaceae, and low Streptococcus levels. Interestingly, once cluster 3 was reached by an individual at any time point, samples from later time points tended to stay within cluster 3. In contrast, cluster 2 tended to contain earlier samples and most individuals with cluster 2 membership early progressed to cluster 3 later. The high predominance of Klebsiella OTU2 and Streptococcus OTU4 in cluster 2 was striking. Both OTUs may contain facultative pathogens, and they are likely to provide a substantially different environment in the developing infant gut than bifidobacteria, which are considered the optimal colonizers of infants (27, 28). Cluster 1 membership did not follow such a strong pattern across time points, although it was slightly more frequent at early time points. High Firmicutes levels were characteristic of this cluster and have been observed in the early microbiota of premature infants (25).
Cluster 3 was reached relatively later in infants delivered by caesarean section (Fig. 2A). Lower or later colonization by bifidobacteria in babies delivered by cesarean section is consistent with earlier studies (29). By 6 months, no differences in microbiotas were detected between infants born vaginally and those delivered by caesarean section. This agrees with other studies (30) but does not preclude a long-lasting effect on the immune system or intestinal barrier function of differences in the early microbiota driven by the delivery mode (18, 31–34).
Previous studies have found that preterm infants have a microbiota composition very different from that of full-term infants (35–39) and linked this with detrimental outcomes, such as necrotizing enterocolitis. Even though all of our infants were born at term, the duration of gestation at birth was significantly associated with the infant’s microbiota profile over time, with those born after a shorter duration of gestation tending to reach cluster 3 later (Fig. 2D, P = 0.0163). A relationship between variation in gestation duration within the normal range and the microbiota profile is remarkable; however, we note that Karlsson et al. (40) also found that the abundance of bacterial groups was associated with a birth weight within the normal range. A shorter gestation duration may be associated with subtle gut immaturity, even within the normal range, with negative consequences for the gut microbiota; this is consistent with recent results showing that early term infants (gestational age of 37 to 38 weeks) show suboptimal outcomes (41, 42). In agreement with reference 24, we found that a shorter gestation duration was associated with a lag in microbiota progression.
An important finding of our study was that reaching cluster 3 later in infancy was predictive of lower adiposity at 18 months of age (Fig. 2F). This may be due to lasting effects of a differential early microbiota or to causative factors affecting both body composition and the microbiota. Infants who had unusually high Streptococcus abundances at month 6 displayed a tendency for a greater increase in adiposity (Fig. 2G); this may be particularly relevant in the light of recent findings linking the extent of adiposity increase in infancy, rather than absolute values of adiposity, to suboptimal health outcomes (43). Adult obesity has been associated with an altered microbiota profile (8). Recent findings obtained with mice show that low-dose penicillin limited to early life acts through an altered microbiota to induce sustained effects on body composition (18). However, the very different microbiotas of mice and human infants make it difficult to apply observations on particular taxa directly to human adiposity.
The infant feeding mode was not significantly associated with the time to cluster 3, although we did find limited support (P = 0.09) for the association between breastfeeding status and the abundance of members of the class Gammaproteobacteria (at month 6 in our data) shown in reference 24. In addition, multiple other taxa were nominally significantly (P < 0.05) associated with the feeding pattern at the univariate level (see Table S2 in the supplemental material). However, the majority of the infants in our study were fed a mixture of breast milk and formula at early time points (49 mixed, 10 exclusively formula fed, and 14 exclusively breastfed at day 3); moreover, the majority (n = 47) were exclusively formula fed at 6 months. Our study therefore lacked the discriminatory power necessary to detect effects of infant feeding. Many other groups have noted differences between the microbiotas of breastfed and formula-fed infants (44).
The concept of microbiota maturity was recently introduced by Subramanian et al. (45), who found that an immature microbiota was clearly associated with acute malnutrition. We note that the 24 most age-discriminatory taxa in the analysis of Subramanian et al. included Bifidobacterium and Streptococcus species, which were important discriminant taxa in our clustering. This similarity was seen despite the radically different circumstances and age ranges of the children included in the study of Subramanian et al. and ours.
Reaching cluster 3 early was associated in our study with factors that are thought to be favorable Table 2 (i.e., longer duration of gestation, vaginal delivery, and adiposity in line with global medians). In contrast, caesarean section delivery, a shorter gestational duration, and relatively low adiposity at 18 months were all associated with a slight microbiota delay Table 2; suggesting that the delay is suboptimal. This is somewhat consistent with the profound gut microbiota immaturity observed by Subramanian et al. in malnourished children.
TABLE 2 .
Characteristics of infants who reach a month 6-like microbiota earlier or later
| Point at which infants reached “month 6-like” microbiota | Tendency of: |
||||||
|---|---|---|---|---|---|---|---|
| Enterobacteriaceae levels | Streptococcus levels | Bifidobacterium levels | Collinsella levels | Delivery mode | Gestation duration | Skinfold thickness at 18 mo | |
| Later | Higher | Higher | Caesarian section | Shorter | Thinner | ||
| Earlier | Higher | Higher | Vaginal | Longer | Typical | ||
A limitation of this study was the resolution of our sequence depth. We did not have information on which prokaryotic genes were expressed, and sequence depth limits the detection of nonabundant taxa (34). A final caveat is that fecal sampling is not a wholly accurate proxy for the gastric and intestinal microbiota. It tends to undersample biodiversity and does not reflect site-specific differences (46). We chose to use the results from sequencing of the V456 region of the rRNA, as these yielded a more complete data set with less missing data; however, V123 was also sequenced and yielded very similar results (see Fig. S4 and Table S3 in the supplemental material).
To our knowledge, this study is the largest of its type in terms of both including 75 individuals and sampling them longitudinally across four time points. The 75 individuals included had normal phenotypes with no extreme values of gestational age or adiposity; the majority received mixed breastfeeding and formula feeding. However, the rate at which a more anaerobic microbiota was obtained correlated with the duration of gestation at birth and the mode of delivery, as well as measures of infant body composition.
Conclusion.
An earlier acquisition of an anaerobic microbiome dominated by Bifidobacterium and Collinsella compared to Enterobacteriaceae and Streptococcus within the first 6 months of life is associated with a longer gestation duration at birth, vaginal delivery, and typical adiposity at 18 months of age.
MATERIALS AND METHODS
Subjects, sample collection, and DNA extraction.
Fecal samples were collected at day 3, week 3, month 3, and month 6 from 75 infants in the GUSTO birth cohort, which includes members of the three main ethnic groups in Singapore, i.e., Chinese, Indian, and Malay; a detailed description of the GUSTO cohort has been published elsewhere (21). This study was approved by both the National Healthcare Group Domain Specific Review Board (reference no. D/09/021) and the Sing Health Centralized Institutional Review Board (reference no. 2009/280/D). All of the participants who provided data gave informed written consent to donate their children’s stool samples prior to their participation. Table 1 shows the maternal, delivery, and infant size/growth characteristics of the subjects. Samples were stored under anaerobic conditions until freezing, which took place a maximum of 10 h after sample emission. All samples were stored at −80 C. Total DNA was extracted with the QIAamp DNA stool minikit (Qiagen, Germany) in accordance with the manufacturer’s instructions, except for the addition of a series of mechanical disruption steps (11 × 45 s) with a FastPrep apparatus and Lysing Matrix B tubes (MP Biochemicals, Santa Ana, CA) (50).
DNA sequencing.
Two loci covering variable regions V1, V2, and V3 (V123) and V4, V5, and V6 (V456) of the 16S rRNA gene were used to characterize the microbiota by the barcoded-primer approach to multiplex pyrosequencing as described in reference 47 and detailed in the supplemental material. Both sets of primers were selected to ensure the optimal coverage of bacterial phylogenetic diversity, in particular, bifidobacteria. A total of 946,541 good-quality sequencing reads were obtained. The average numbers of reads per sample were 1,494 and 1,667 for the V123 and V456 regions, respectively.
Data processing
Data processing was performed according to reference 48, and it is described in detail in the supplemental material. Briefly, denoising was performed with the PyroNoise algorithm, and the sequencing reads were quality checked by using stringent criteria (zero ambiguities, one mismatch permitted in a bar code; reads were trimmed to 250 bp before alignment) and chimera checked. The labels for OTUs were obtained by Ribosomal Database Project (RDP) classification of a representative sequence of each OTU. One dominant OTU belonging to the family Enterobacteriaceae was labeled as unclassified at the genus level. A BLAST search performed with a representative sequence of this OTU revealed that it showed 100% identity with several type strains of members of the family Enterobacteriaceae. Representative sequence of the corresponding OTU in region V123 yielded a 100% match to Klebsiella pneumoniae and more distant matches to other sequences. The analysis was performed primarily with the reads from region V456.
Univariate analysis with respect to phenotypes.
Groups were compared by Student’s t tests assuming homoscedasticity. RDP and OTU data were filtered by setting all singleton read data (i.e., read depth = 1) to zero and removing taxa that had reads detected in only one sample. For taxa at all phylogenetic levels and at each time point, linear regression was run for relative taxon abundance against the continuous phenotypes shown in Table 1; analyses of variance (ANOVAs) were run for relative taxon abundance against the categorical phenotypes. Mode of feeding was divided into the categories exclusively breastfed, mixed, and exclusively formula fed at the four time points (Table 1). False-discovery-rate-corrected P values were estimated as described by Benjamini and Hochberg (49) for all phenotypic comparisons conducted at each phylogenetic level.
Detection of clusters.
A combined table containing data for subjects at all time points and all of the bacteria at different taxon levels (phylum, class, order, family, genus, and OTU) was produced. The data were normalized by mean value across taxa and samples [x/(1/n mean)]. Hierarchical clustering was performed by the unweighted-pair group method using average linkages (UPGMA) with euclidean distance. The sample dendrogram was pruned at distance 238 (chosen by visual inspection) to reveal three deep-rooted clusters. Cluster 1 (high in Firmicutes and its constituent genera Streptococcus and Lactobacillus) contained 36 samples, cluster 2 (high in members of the family Enterobacteriaceae) contained 61 samples, and cluster 3 (high in Bifidobacterium and Collinsella) contained 181 samples
Assignment of subjects to temporal groups and analysis with respect to phenotypes.
Using the cluster definitions above, we next categorized each subject by the time point of first assignment to cluster 3. For example, if subject x was in cluster 2 at day3, cluster 2 at week 3, cluster 3 at month 3, and cluster 3 at month 6, the assigned value was 3 months. In this manner, 61 of the 75 subjects were assigned a time point at which they reached (and remained in) cluster 3. Three subjects never reached cluster 3, seven subjects were in cluster 3 during the time course but reverted to another cluster by month 6, and four subjects had missing data penultimate to the time point where cluster 3 was first seen; therefore, a call could not be made. The time point at which the subject reached cluster 3 was then tested against the environmental and phenotypic data by using either a chi-square test or ANOVA, as appropriate.
SUPPLEMENTAL MATERIAL
Summary view of microbiotas. Summary data are displayed across time points (columns) and phylogenetic levels (rows). At the phylum level, Proteobacteria are yellow, Actinobacteria are red, Firmicutes are blue, and Bacteroidetes are green. The same color scheme is maintained for constituent taxa. The size of a node is proportional to the relative percent abundance (≥1 relative percent shown here). Positive correlations between taxa are depicted by green edges, while negative correlations are red, with the thickness of the edge proportional to the absolute amount of correlation. Download
Summary view of interindividual variation. Seventy-five individuals are displayed as columns across the four panels representing four time points. Bars are proportional to the relative abundances of phyla. White bars denote samples that were removed during preprocessing. Phylum colors are as follows: red, Actinobacteria; orange, Bacteroidetes; purple, Firmicutes; brown, Proteobacteria. Download
Larger version of Fig. 1A including readable taxon names on the x axis. Download
Clustering of V123 region data reveals the presence of three clusters similar to those reported for the V456 region data. Pie charts show a similar relationship between cluster membership and the time point. Download
Multivariate regression of associations featured in Fig. 2. P values of <0.05 are red.
Associations with P values of <0.05 for feeding modes and taxon levels.
Time points at which cluster 3 was reached on the basis of V123 versus V456 region data. It was possible to make a call for only 35 samples on the basis of region V123 data. Twenty-eight of these agree with calls based on V456 region data; seven of these differ by one time point.
ACKNOWLEDGMENTS
The GUSTO Study Group includes Pratibha Agarwal, Arijit Biswas, Choon Looi Bong, Birit F. P. Broekman, Shirong Cai, Jerry Kok Yen Chan, Yiong Huak Chan, Cornelia Yin Ing Chee, Helen Y. H. Chen, Yin Bun Cheung, Audrey Chia, Amutha Chinnadurai, Chai Kiat Chng, Mary Foong-Fong Chong, Shang Chee Chong, Mei Chien Chua, Chun Ming Ding, Eric Andrew Finkelstein, Doris Fok, Marielle Fortier, Anne Eng Neo Goh, Yam Thiam Daniel Goh, Joshua J. Gooley, Wee Meng Han, Mark Hanson, Christiani Jeyakumar Henry, Chin-Ying Hsu, Hazel Inskip, Jeevesh Kapur, Kenneth Kwek, Ivy Yee-Man Lau, Bee Wah Lee, Ngee Lek, Sok Bee Lim, Yen-Ling Low, Iliana Magiati, Lourdes Mary Daniel, Cheryl Ngo, Krishnamoorthy Naiduvaje, Wei Wei Pang, Anqi Qiu, Boon Long Quah, Victor Samuel Rajadurai, Mary Rauff, Salome A. Rebello, Jenny L. Richmond, Anne Rifkin-Graboi, Lynette Pei-Chi Shek, Allan Sheppard, Borys Shuter, Leher Singh, Walter Stunkel, Lin Lin Su, Kok Hian Tan, Oon Hoe Teoh, Hugo P. S. van Bever, Rob M. van Dam, Inez Bik Yun Wong, P. C. Wong, George Seow, and Heong Yeo.
This work was supported by the Translational Clinical Research Flagship Program on Developmental Pathways to Metabolic Disease funded by the National Research Foundation and administered by the National Medical Research Council, Singapore (NMRC/TCR/004-NUS/2008). Additional funding was provided by the Agency for Science and Technology Research (A*STAR) and Nestlé.
OS, CNB, WB, BB and HB are employees of the Nestlé Research Center, a commercial entity that aims to enhance the quality of consumers’ lives through nutrition, health and wellness. Nestle is active in research into prebiotics and probiotics and produces infant formula. KMG and YSC have received reimbursement for speaking at conferences sponsored by companies selling nutritional products, and are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. The other authors have no potential conflicts of interest. A patent in a related field has been submitted (No. 14181275.0).
Footnotes
Citation Dogra S, Sakwinska O, Soh S, Ngom-Bru C, Brück WM, Berger B, Brüssow H, Lee YS, Yap F, Chong Y, Godfrey KM, Holbrook JD. 2015. Dynamics of infant gut microbiota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. mBio 6(1):e02419-14. doi:10.1128/mBio.02419-14.
Contributor Information
Willem M. de Vos, Wageningen University.
Maria Gloria Dominguez Bello, New York University School of Medicine.
REFERENCES
- 1.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. 2013. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 2.Zheng X, Zhao A, Xie G, Chi Y, Zhao L, Li H, Wang C, Bao Y, Jia W, Luther M, Su M, Nicholson JK, Jia W. 2013. Melamine-induced renal toxicity is mediated by the gut microbiota. Sci Transl Med 5:172ra122. doi: 10.1126/scitranslmed.3005114. [DOI] [PubMed] [Google Scholar]
- 3.Collins SM, Surette M, Bercik P. 2012. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 5.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 6.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. 2007. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A 104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joyce SA, Gahan CG. 2014. The gut microbiota and the metabolic health of the host. Curr Opin Gastroenterol 30:120–127. doi: 10.1097/MOG.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 9.Burcelin R, Garidou L, Pomié C. 2012. Immuno-microbiota cross and talk: the new paradigm of metabolic diseases. Semin Immunol 24:67–74. doi: 10.1016/j.smim.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K. 2012. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 11.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. 2012. Human gut microbiome viewed across age and geography. Nature 486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matamoros S, Gras-Leguen C, Le Vacon F, Potel G, de la Cochetiere MF. 2013. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol 21:167–173. doi: 10.1016/j.tim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Adlerberth I, Wold AE. 2009. Establishment of the gut microbiota in Western infants. Acta Paediatr 98:229–238. doi: 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 14.Ajslev TA, Andersen CS, Gamborg M, Sørensen TI, Jess T. 2011. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond) 35:522–529. doi: 10.1038/ijo.2011.27. [DOI] [PubMed] [Google Scholar]
- 15.Murphy R, Stewart AW, Braithwaite I, Beasley R, Hancox RJ, Mitchell EA, ISAAC Phase Three Study Group . 2014. Antibiotic treatment during infancy and increased body mass index in boys: an international cross-sectional study. Int J Obes (Lond) 38:1115-1119. doi: 10.1038/ijo.2013.218. [DOI] [PubMed] [Google Scholar]
- 16.Blustein J, Attina T, Liu M, Ryan AM, Cox LM, Blaser MJ, Trasande L. 2013. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int J Obes (Lond) 37:900–906. doi: 10.1038/ijo.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huh SY, Rifas-Shiman SL, Zera CA, Edwards JWR, Oken E, Weiss ST, Gillman MW. 2012. Delivery by caesarean section and risk of obesity in preschool age children: a prospective cohort study. Arch Dis Child 97:610–616. doi: 10.1136/archdischild-2011-301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox L, Yamanishi S, Sohn J, Alekseyenko A, Leung J, Cho I, Kim S, Li H, Gao Z, Mahana D, Zárate Rodriguez J, Rogers A, Robine N, Loke P, Blaser M. 2014. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, Heederik D, Piarroux R, von Mutius E, GABRIELA Transregio 22 Study Group . 2011. Exposure to environmental microorganisms and childhood asthma. N Engl J Med 364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 20.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bønnelykke K, Brasholt M, Heltberg A, Vissing NH, Thorsen SV, Stage M, Pipper CB. 2007. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 21.Soh S-, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, Stunkel W, Holbrook JD, Kwek K, Chong Y-, Saw SM, Sheppard A, Chinnadurai A, Ferguson-Smith A, Goh AEN, Biswas A, Chia A, Leutscher-Broekman B, Shuter B, Cai S. 2014. Cohort profile: growing up in Singapore towards healthy outcomes (GUSTO) birth cohort study. Int J Epidemiol 43:1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 22.Anonymous. 2007. WHO child growth standards. World Health Organization, Geneva, Switzerland: http://www.who.int/childgrowth/standards/Technical_report.pdf. [Google Scholar]
- 23.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, Sears MR, Becker AB, Scott JA, Kozyrskyj AL. 2013. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. Can Med Assoc J 185:385–394. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, Stevens HJ, Bennett WE Jr., Shaikh N, Linneman LA, Hoffmann JA, Hamvas A, Deych E, Shands BA, Shannon WD, Tarr PI. 2014. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci U S A 111:12522–12527. doi: 10.1073/pnas.1409497111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jost T, Lacroix C, Braegger CP, Chassard C. 2012. New insights in gut microbiota establishment in healthy breast fed neonates. PLoS One 7:e44595. doi: 10.1371/journal.pone.0044595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. 2011. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 108:1478–1485. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oozeer R, van Limpt K, Ludwig T, Ben Amor K, Martin R, Wind RD, Boehm G, Knol J. 2013. Intestinal microbiology in early life: specific prebiotics can have similar functionalities as human-milk oligosaccharides. Am J Clin Nutr 98:561S–571S. doi: 10.3945/ajcn.112.038893. [DOI] [PubMed] [Google Scholar]
- 28.Patel RM, Denning PW. 2013. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: what is the current evidence? Clin Perinatol 40:11–25. doi: 10.1016/j.clp.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. 2010. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev 86(Suppl 1):13–15. doi: 10.1016/j.earlhumdev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Trosvik P, Stenseth NC, Rudi K. 2010. Convergent temporal dynamics of the human infant gut microbiota. ISME J 4:151–158. doi: 10.1038/ismej.2009.96. [DOI] [PubMed] [Google Scholar]
- 31.Collado MC, Cernada M, Baüerl C, Vento M, Pérez-Martínez G. 2012. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes 3:352–365. doi: 10.4161/gmic.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerr CA, Grice DM, Tran CD, Bauer DC, Li D, Hendry P, Hannan GN. 2014. Early life events influence whole-of-life metabolic health via gut microflora and gut permeability. Crit Rev Microbiol. doi: 10.3109/1040841X.2013.837863. [DOI] [PubMed] [Google Scholar]
- 33.Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S, Hamelmann E. 2013. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol 132:601–607.e8. doi: 10.1016/j.jaci.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 34.van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, Reijmerink NE, Dompeling E, van den Brandt PA, Ferreira I, Mommers M, Thijs C. 2011. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol 128:948–955.e1-3. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Sharon I, Morowitz MJ, Thomas BC, Costello EK, Relman DA, Banfield JF. 2013. Time series community genomics analysis reveals rapid shifts in bacterial species, strains, and phage during infant gut colonization. Genome Res 23:111–120. doi: 10.1101/gr.142315.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morowitz MJ, Denef VJ, Costello EK, Thomas BC, Poroyko V, Relman DA, Banfield JF. 2011. Strain-resolved community genomic analysis of gut microbial colonization in a premature infant. Proc Natl Acad Sci U S A 108:1128–1133. doi: 10.1073/pnas.1010992108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moles L, Gómez M, Heilig H, Bustos G, Fuentes S, de Vos W, Fernández L, Rodríguez JM, Jiménez E. 2013. Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS One 8:e66986. doi: 10.1371/journal.pone.0066986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arboleya S, Binetti A, Salazar N, Fernández N, Solís G, Hernández-Barranco A, Margolles A, los Reyes-Gavilan CG, Gueimonde M. 2012. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol Ecol 79:763–772. doi: 10.1111/j.1574-6941.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 39.Arboleya S, Ang L, Margolles A, Yiyuan L, Dongya Z, Liang X, Solís G, Fernández N, de los Reyes-Gavilán CG, Gueimonde M. 2012. Deep 16S rRNA metagenomics and quantitative PCR analyses of the premature infant fecal microbiota. Anaerobe 18:378–380. doi: 10.1016/j.anaerobe.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Karlsson CLJ, Molin G, Cilio CM, Ahrné S. 2011. The pioneer gut microbiota in human neonates vaginally born at term—a pilot study. Pediatr Res 70:282–286. doi: 10.1203/PDR.0b013e318225f765. [DOI] [PubMed] [Google Scholar]
- 41.Sengupta S, Carrion V, Shelton J, Wynn RJ, Ryan RM, Singhal K, Lakshminrusimha S. 2013. Adverse neonatal outcomes associated with early-term birth. JAMA Pediatr 167:1053–1059. doi: 10.1001/jamapediatrics.2013.2581. [DOI] [PubMed] [Google Scholar]
- 42.Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, Ji Y, Hong X, Walker SO, Caruso D, Pearson C, Wang M, Zuckerman B, Cheng TL, Wang X. 2014. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA 311:587–596. doi: 10.1001/jama.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howe LD, Chaturvedi N, Lawlor DA, Ferreira DLS, Fraser A, Davey Smith G, Tilling K, Hughes AD. 2014. Rapid increases in infant adiposity and overweight/obesity in childhood are associated with higher central and brachial blood pressure in early adulthood. J Hypertens 32:1789–1796. doi: 10.1097/HJH.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roger LC, McCartney AL. 2010. Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology 156:3317–3328. doi: 10.1099/mic.0.041913-0. [DOI] [PubMed] [Google Scholar]
- 45.Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, Barratt MJ, VanArendonk LG, Zhang Q, Province MA, Petri WA Jr., Ahmed T, Gordon JI. 2014. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510:417–421. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romano-Keeler J, Moore DJ, Wang C, Brucker RM, Fonnesbeck C, Slaughter JC, Li H, Curran DP, Meng S, Correa H, Lovvorn HN III, Tang Y, Bordenstein S, George AL Jr., Weitkamp J. 2014. Early life establishment of site-specific microbial communities in the gut. Gut Microbes 5:192–201. doi: 10.4161/gmic.28442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez M, Darimont C, Drapeau V, Emady-Azar S, Lepage M, Rezzonico E, Ngom-Bru C, Berger B, Philippe L, Ammon-Zuffrey C, Leone P, Chevrier G, St-Amand E, Marette A, Doré J, Tremblay A. 2014. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr 111:1507–1519. doi: 10.1017/S0007114513003875. [DOI] [PubMed] [Google Scholar]
- 48.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Stat Methodol 57:289–300. [Google Scholar]
- 50.Junick J, Blaut M. 2012. Quantification of human fecal bifidobacterium species by use of quantitative real-time PCR analysis targeting the groEL gene. App Environ Microbiol 78:2613–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary view of microbiotas. Summary data are displayed across time points (columns) and phylogenetic levels (rows). At the phylum level, Proteobacteria are yellow, Actinobacteria are red, Firmicutes are blue, and Bacteroidetes are green. The same color scheme is maintained for constituent taxa. The size of a node is proportional to the relative percent abundance (≥1 relative percent shown here). Positive correlations between taxa are depicted by green edges, while negative correlations are red, with the thickness of the edge proportional to the absolute amount of correlation. Download
Summary view of interindividual variation. Seventy-five individuals are displayed as columns across the four panels representing four time points. Bars are proportional to the relative abundances of phyla. White bars denote samples that were removed during preprocessing. Phylum colors are as follows: red, Actinobacteria; orange, Bacteroidetes; purple, Firmicutes; brown, Proteobacteria. Download
Larger version of Fig. 1A including readable taxon names on the x axis. Download
Clustering of V123 region data reveals the presence of three clusters similar to those reported for the V456 region data. Pie charts show a similar relationship between cluster membership and the time point. Download
Multivariate regression of associations featured in Fig. 2. P values of <0.05 are red.
Associations with P values of <0.05 for feeding modes and taxon levels.
Time points at which cluster 3 was reached on the basis of V123 versus V456 region data. It was possible to make a call for only 35 samples on the basis of region V123 data. Twenty-eight of these agree with calls based on V456 region data; seven of these differ by one time point.