Figure 2.
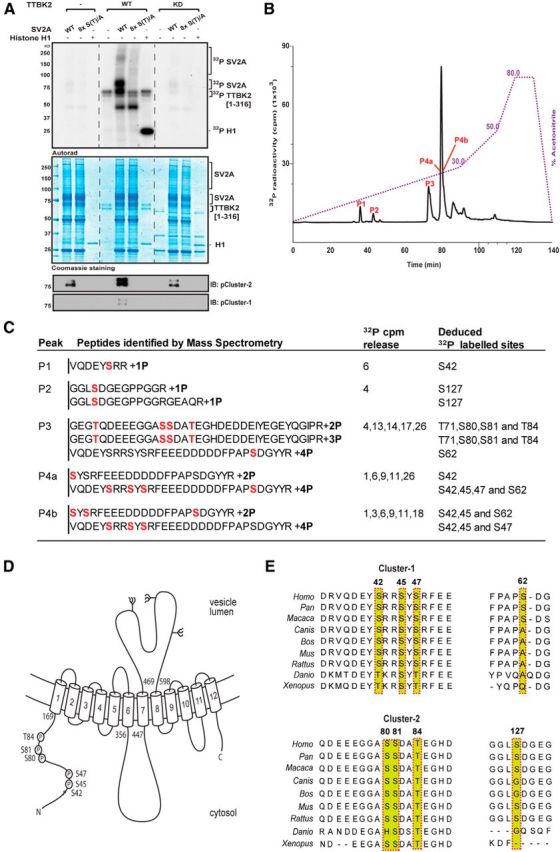
Identification of TTBK2 phosphorylation sites on SV2A. A, HEK293 cells were transfected with a construct encoding WT Flag–SV2A or a mutant Flag–SV2A in which all eight phosphorylation sites identified in C, namely Ser42, Ser45, Ser47, Ser62, Ser80, Ser81, Thr84, and Ser127, are mutated to Ala [termed 8× S(T)/A]. Thirty-six hours after transfection, cells were lysed, and WT and mutant Flag–SV2A was immunoprecipitated and incubated with recombinant WT TTBK2[1–316] or kinase inactive (KD) TTBK2[D163A, 1–316] and Mg2–[-32P]ATP for 60 min. Samples were subjected to electrophoresis on a polyacrylamide gel that was stained with colloidal Coomassie blue and autoradiographed. Reactions were also subjected to immunoblot analysis with the indicated phospho-specific antibodies that recognize phosphorylated Cluster-1 or Cluster-2 sites. It should be noted that a low level of unidentified kinase(s) copurifies with Flag–SV2A from HEK293 cells, which phosphorylates it to a low level in the absence of TTBK2. B, Full-length Flag–SV2A was incubated with WT TTBK2[1–316] in the presence of Mg2+–[γ-32P]ATP for 60 min as in A. Phosphorylated SV2A was digested with trypsin, and peptides were separated by reverse-phase HPLC on a C18 column. The peaks containing the 32P-labeled phosphopeptides are marked as in the subdivisions of C. C, Summary of the mass spectrometry and solid-phase Edman sequencing data obtained after analysis of the peak fractions. The deduced amino acid sequence of each peptide is shown, and the deduced 32P-labeled site is indicated in red. Note that, as SV2A was isolated from HEK293 cells, certain peptides are pre-phosphorylated by endogenous kinase(s). D, Domain structure of SV2A with the position of eight residues phosphorylated by TTBK2 indicated. All numbering refers to the human sequence. E, Sequence alignment of the residues of SV2A that encompass the TTBK2 phosphorylation sites from the indicated species. Residues highlighted indicated in red comprise the phosphorylation sites.
