Figure 9.
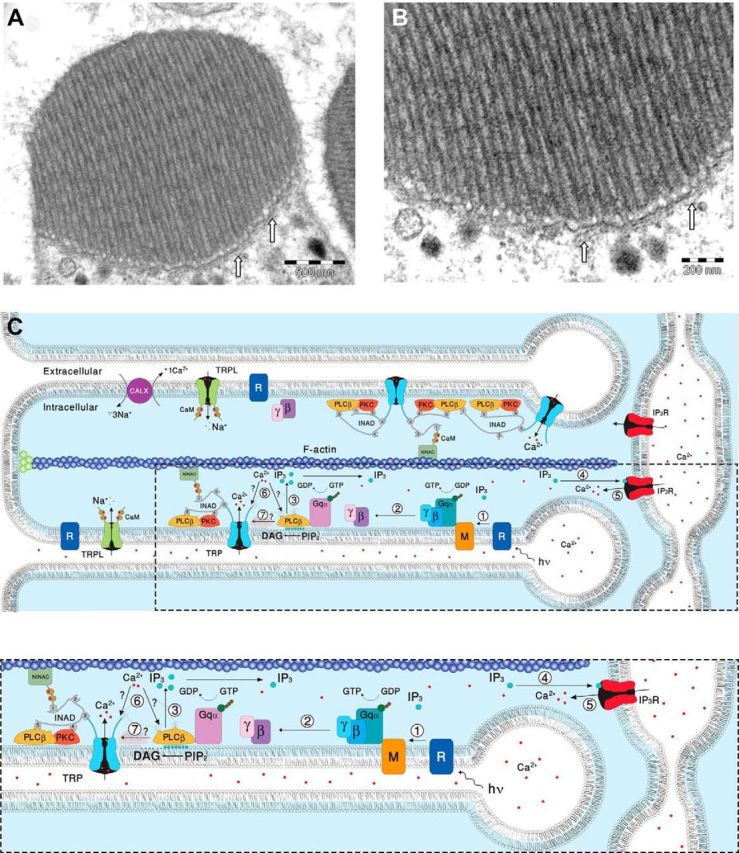
EM pictures and a diagram showing structural features of the signaling compartment and the localization of the signaling molecules. Top, TEM showing the microvilli composing dark-adapted WT rhabdomere. A, TEM of a cross section of a rhabdomere, which is composed of tightly packed microvilli. The elongated membrane vesicles virtually touching the base of the microvilli (arrows) are extensions of the smooth ER called SMC and constitute the IP3-sensitive Ca2+ stores. B, Higher magnification of the rhabdomere at the base of the microvilli, showing the SMC (arrows). C, A diagram showing the molecular components of the signal transduction cascade of Drosophila:,1, Upon absorption of a photon (hν), rhodopsin (R) is converted into metarhodopsin (M). 2, The R-to-M photoconversion leads to the activation of heterotrimeric G-protein(Gqα,β,γ) by promoting the GDP-to-GTP exchange. 3, The GDP-to-GTP exchange in turn leads to activation of PLCβ, which hydrolyzes PIP2 into the soluble IP3 and the membrane-bound DAG. 4, Subsequently, IP3 molecules (blue dots) diffuse along the microvillus and bind to the IP3 receptor (IP3R) located at the SMC. 5, Binding of IP3 to the IP3R causes release of Ca2+ (red dots) from the SMC and its diffusion back into the microvillus followed by binding of Ca2+ to both PLCβ and the TRP channel. 6, This binding either facilitates the catalytic activity of PLCβ (?) or reduces the threshold of TRP channel activation (?, see Fig. 8). 7, Two classes of light-sensitive channels, the TRP and TRPL, open by a still unknown mechanism (?) following PLCβ activation. The TRP and TRPL channel openings lead to elevation of cellular Ca2+. Elevation of DAG and Ca2+ promote eye-specific PKC activity, which regulates channel activity. PLCβ, PKC, and the TRP ion channel form a supramolecular complex with the scaffolding protein INAD, which is bound to the F-actin cytoskeleton via the NINAC protein. Bottom, Magnification of the box marked by dotted lines in the top diagram.
