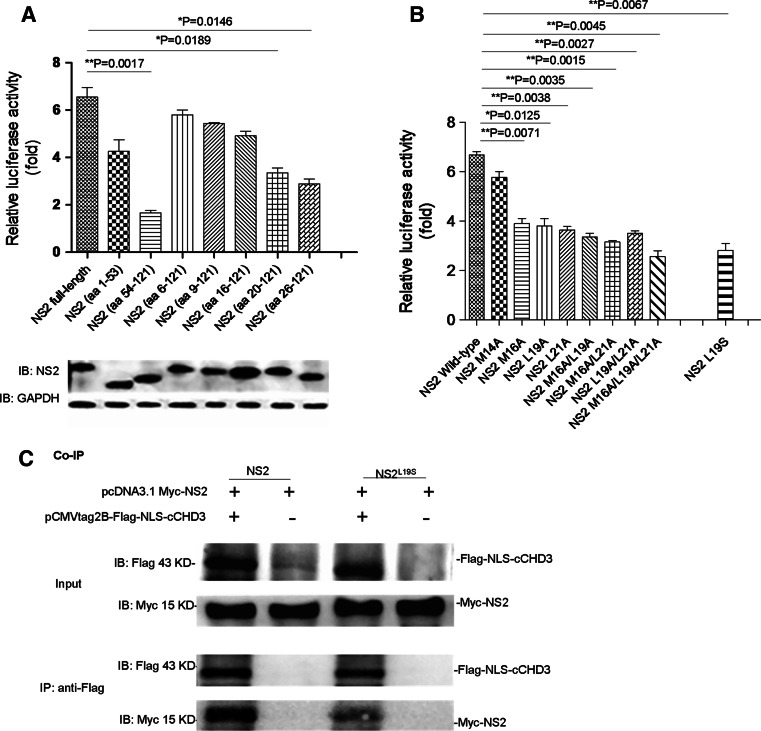
Fig. 3.
The key amino acids (M16, M19 and L21) of NS2 were involved in NS2–CHD3 interaction. a The NS2 N-terminus containing aa 16–20 mediated interactions with CHD3. Top COS-1 cells were cotransfected with pACT-cCHD3, pG5luc and pBIND-NS2 or pBIND-NS2-truncations. The strength of the interaction between cCHD3 and the NS2 truncations was assayed via M2H assay 24 h later. The interaction between CHD3 and the NS2 truncations was normalized to the self-activation of the NS2 truncations (co-transfection of the pBIND-NS2 truncations and pACT plasmid). The results are shown as the mean ± SD for three independent experiments (*p < 0.05, **p < 0.01, n = 3). Bottom the expression level of the NS2 truncations was detected with an anti-NS2 polyclonal antibody. GAPDH served as a protein loading control. b The NES of NS2 mediates interactions with CHD3. COS-1 cells were cotransfected with pACT-cCHD3, pG5luc and pBIND-NS2 or pBIND-NS2 mutants, and the strength of the interaction was assayed via M2H assay as above. The results are shown as the mean ± SD (*p < 0.05, **p < 0.01, n = 3). c NS2 (L19S) bound cCHD3 but weaker than NS2 did in Co-IP assay