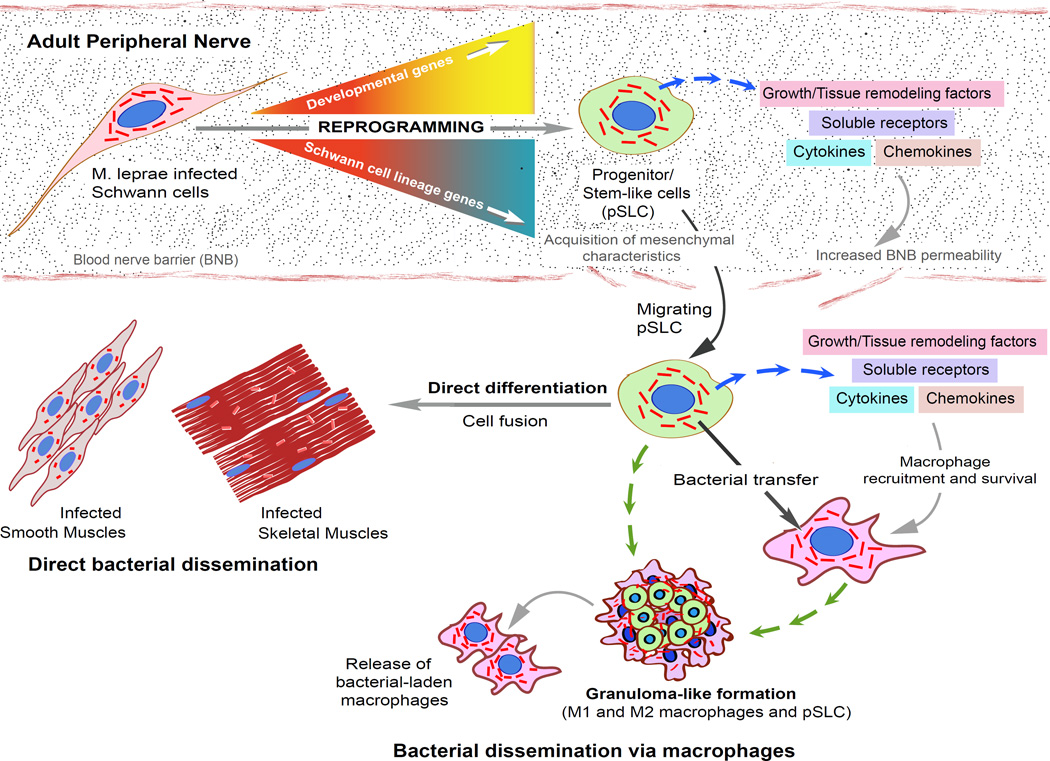
Figure 3. The proposed model for reprogramming adult Schwann cells to stem cell-like cells by intracellular M. leprae and subsequent events leading to dissemination of infection.
Schwann cells in the adult peripheral nerves infected with ML undergo a reprogramming process that convert Schwann cells to pSLC by turning off Schwann cell differentiation/myelination program-associated genes and upregulating embryonic genes of mesenchymal and neural crest development. Reprogramming renders pSLC to acquire migratory properties and immunomodulatory characteristics - releasing numerous chemokines, cytokines and growth/remodeling factors, which not only increased permeability of blood nerve barrier (BNB) but also attract macrophages. Acquired migratory properties promote ML-laden pSLC to exit breached BNB and disseminate to other preferred tissue niches such as smooth muscles and skeletal muscles where they can undergo direct differentiation, and thus transfer bacteria passively to these tissues. Chemoattractants released from pSLC recruit macrophages, transfer ML and form typical granuloma-like structures, which then release bacterial-laden macrophages, a mechanism by which reprogrammed cells may channel bacterial dissemination via systemic routes.