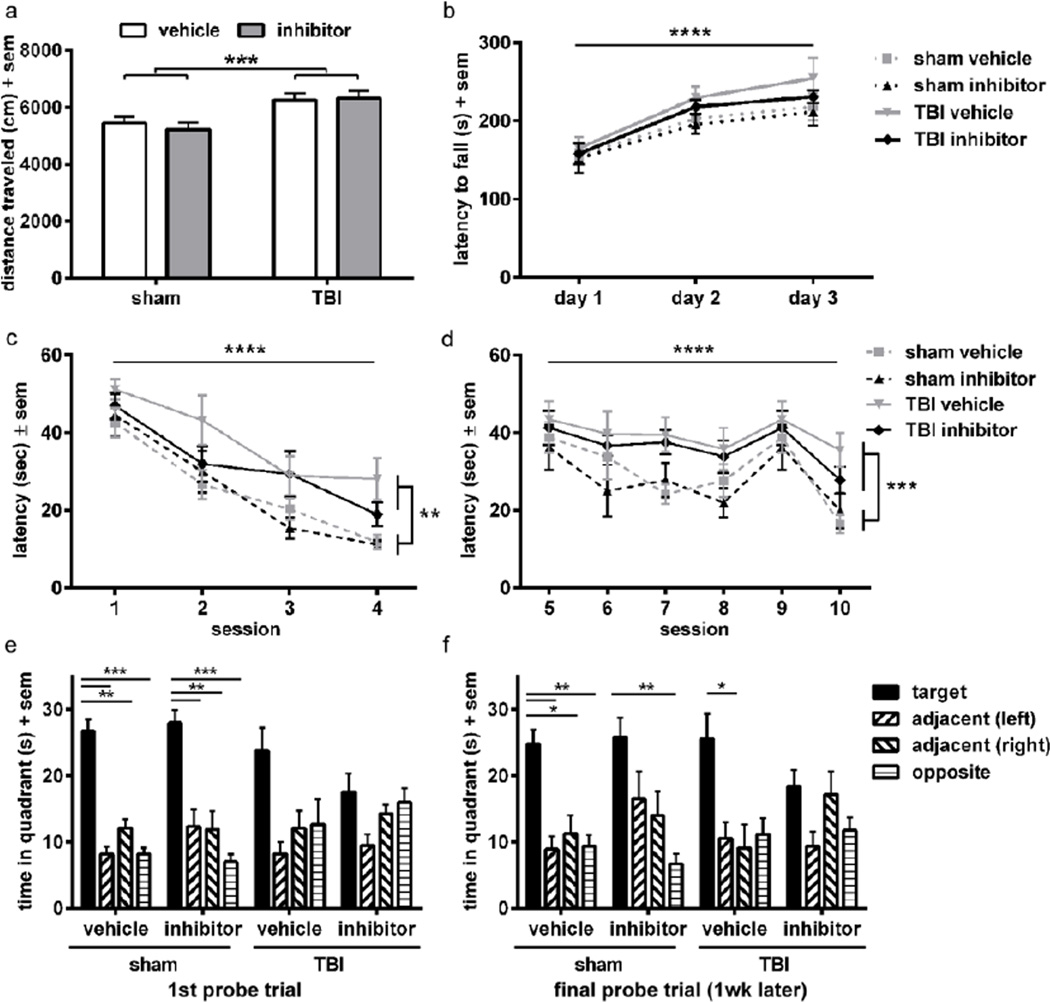
Figure 6. Treatment with the NE inhibitor ZN200,355 does not alter long-term functional outcomes after TBI at p21.
TBI mice show hyperactivity in the open field as compared to sham controls (a; 2-way ANOVA effect of injury ***p<0.001), however, this was not affected by treatment with the NE inhibitor. Although all mice showed improvement over time on the accelerating rotarod (b; 2-way RM ANOVA effect of time ****p<0.0001), none of the groups differed from each other. Spatial task learning was assessed in the MWM visible sessions (c), revealing an impairment in TBI mice compared to sham (linear mixed effects ANOVA effect of injury **p<0.01), although all groups showed improvement (reduced latency to the platform) over sequential sessions (effect of time ****p<0.0001). Similarly in the MWM hidden platform sessions (d), brain-injured mice exhibited a higher latency to reach the platform compared to sham controls overall (effect of injury ***p<0.001), although all groups again showed improvement across sessions (effect of time ****p<0.0001). No differences were observed between vehicle and NE inhibitor-treated mice in either the visible or hidden platform sessions of the MWM. A probe trial conducted at the end of the testing week (e) revealed a preference for the target quadrant in both vehicle and NE inhibitor-treated sham control mice (***p<0.001 and **p<0.01 from Dunnett’s analyses after 1-way RM ANOVAs within each group), whereas TBI mice did not show such a preference, indicating memory retention deficits in brain-injured mice. When tested again one week later (f), vehicle and NE inhibitor-treated sham groups as well as the vehicle TBI mice showed memory retention, whereas NE inhibitor-treated TBI mice did not (**p<0.01 and *p<0.05). n=9–10/group.