Abstract
Background
Isolation of the pulmonary veins (PVs) for the treatment of atrial fibrillation (AF) is often supplemented with linear lesions within the left atrium (LA). However, there are conflicting data on the effects of creating a roof line (RL) joining the superior PVs in paroxysmal atrial fibrillation (PAF).
Methods and Results
A cohort of 120 patients with drug‐refractory PAF referred for ablation were prospectively randomized into 2 strategies: (1) PV isolation in combination with RL ablation (LA roof ablation [LARA]‐1: 59 patients) or (2) PV isolation (LARA‐2: 61 patients). Follow‐up was performed at 1, 3, and 6 months after the procedure and every 6 months thereafter. After a 3‐month blanking period, recurrence was defined as the ocurrence of any atrial tachyarrhythmia lasting ≥30 seconds. PV isolation was achieved in 89% and complete RL block in 81%. RF duration, fluoroscopy, and procedural times were slightly, but not significantly, longer in the LARA‐1 group. After 15±10 months, there was no difference in the arrhythmia‐free survival after a single AF ablation procedure (LARA‐1: 59% vs. LARA‐2: 56% at 12 months; log rank P=0.77). The achievement of complete RL block did not influence the results. The incidence of LA macroreentrant tachycardias was 5.1% in the LARA‐1 group (n=3) versus 8.2% in the LARA‐2 (n=5) (P=ns). Univariate analysis only identified AF duration as a covariate associated with arrhythmia recurrence (hazard ratio, 1.01 [95% confidence interval, 1.002 to 1.012]; P<0.01).
Conclusion
The linear block at the LA roof is not associated with an improved clinical outcome compared with PV isolation alone.
Clinical Trial Registration
URL: ClinicalTrials.gov. Unique identifier: NCT01203241.
Keywords: atrial fibrillation, catheter ablationy, roof line
Introduction
Electrical isolation of the pulmonary veins (PVs) is currently the most commonly employed ablation strategy for the treatment of paroxysmal atrial fibrillation (PAF).1–4 However, in some patients, PV isolation may not be sufficient as a result of greater extension of atrial fibrosis.5 For this reason, the creation of additional linear lesions in the left atrium (LA) has been proposed. These lines include the LA “roof,” the posterior line, and the mitral isthmus.6–12
Still, the role of additional lines remains controversial. The systematic isolation of the posterior wall does not seem to achieve better results.13 In contrast, one single‐centre prospective randomized study suggests a better outcome with the addition of the roof line (RL) in PAF.9
The aim of the present study is to assess whether the LA roof ablation (LARA) adds any benefit to the arrhythmia‐free probability after PAF ablation.
Methods
Between September 2009 and October 2011, 120 consecutive patients admitted for a first catheter ablation for symptomatic, drug‐refractory, paroxysmal PAF were prospectively included in the study. Patients with any treatable cause of atrial fibrillation (AF), contraindication for anticoagulation or LA thrombus, LA anteroposterior diameter >50 mm, moderate or severe mitral valve disease, prosthetic mitral valve, and/or left ventricular (LV) ejection fraction (LVEF) <30% were excluded (Figure 1).
Figure 1.
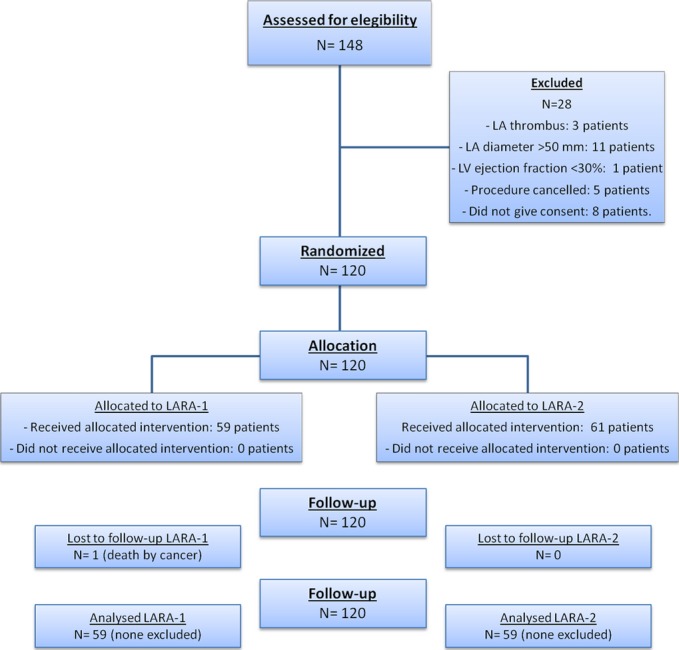
Consort flow chart of the study population. LA indicates left atrium; LAVA, left atrial roof linear ablation; LV, left ventricle.
The study protocol was approved by our hospital's ethics committee, and written informed consent was obtained from all patients according to the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects.
Baseline Assessment
After written informed consent was obtained, a complete clinical evaluation was performed (including physical examination, 12‐lead electrocardiogram (ECG), and laboratory exams). All patients underwent a transthoracic echocardiogram (TTE) 1 to 30 days before the procedure and a multislice computed tomography (CT) or magnetic resonance (MR) to image the LA and PV anatomy. Transesophageal echocardiography was performed 24 to 48 hours before the procedure in patients with indication for chronic oral anticoagulation resulting from a high cardioembolic risk.14 In patients treated with chronic oral anticoagulation, bridging with low‐molecular‐weight heparin (LMWH) was used, which was interrupted 12 hours before the procedure.
Ablation Procedure
The procedure was performed under deep sedation. After dual transseptal access, a bolus of intravenous heparin (50 UI/kg) was administered, with additional boluses to maintain an activated clotting time of >250 seconds and a 20‐pole circular mapping catheter (Lasso®; Biosense Webster, Diamond Bar, CA or Inquiry Optima®; St. Jude Medical, St. Paul, MN) was placed at the PV ostium. Ablation was assisted by a three‐dimensional (3D) nonfluoroscopic mapping system (CARTO XP® or CARTO 3®; Biosense Webster or EnSite NavX®; St. Jude Medical). For accurate visualization of the PVs and other left atrial landmarks, we used image integration of the electroanatomical map with the anatomical information provided by CT or MR imaging (obtained before the procedure) in all patients. Radiofrequency (RF) was delivered by a 3.5‐mm open‐irrigation catheter at a target temperature of 45°C and a maximum output of 40 W (NaviStar® or Celsius®; Biosense Webster). In order to reduce the risk of esophageal complications, power was reduced to 35 W in the posterior wall and RF application was limited to 10 to 20 seconds per point. For all other areas in the LA (including the roof), a 40‐W power limit was used.
Isolation of PVs was achieved by continuous circular lesions around the venoatrial junction of ipsilateral PVs, checking for bidirectional conduction block. An additional line was performed at the carina between ipsilateral veins in case of separate ostia.
After PV electrical isolation, patients were randomly assigned to the 2 ablation strategies (Figure 2): (1) linear ablation at the LA roof (LARA‐1) or (2) no further ablation (LARA‐2). The RL ablation was performed at the most cranial part of the LA, connecting the upper aspect of the right and left PV encircling lesions. RF energy was delivered for 30 to 90 seconds at each point until local potential elimination or double potentials were observed. Completeness of conduction block was confirmed during LA appendage pacing by the online mapping of continuous double potential and an activation detour propagating around the PVs to activate caudocraneally the posterior wall of the LA (Figure 3).9,15 When residual conduction was demonstrated, detailed mapping was performed to identify and ablate gaps in the linear lesion.
Figure 2.
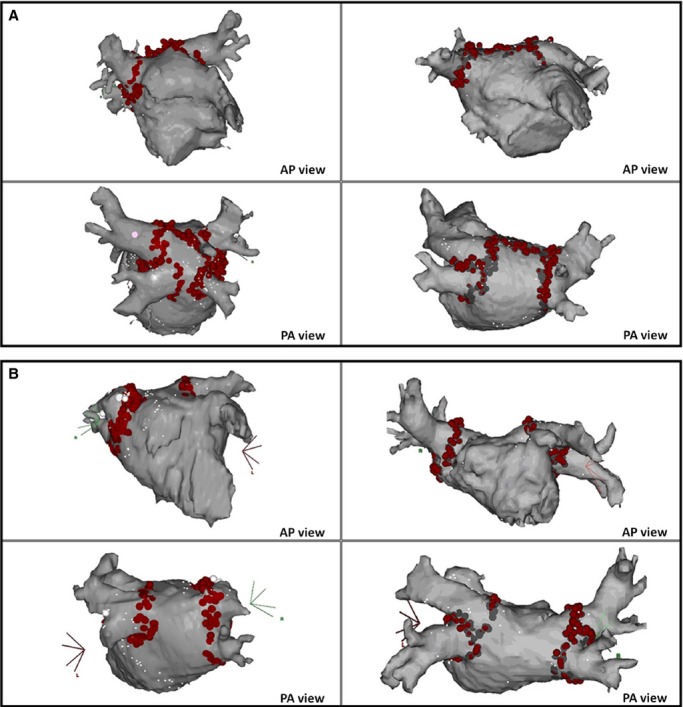
Ablation strategy. A, PV isolation with roof linear ablation (LARA‐1). B, PV isolation without roof linear ablation (LARA‐2). AP indicates anteroposterior; LAVA, left atrial roof linear ablation; PA, posteroanterior; PV, pulmonary veins.
Figure 3.
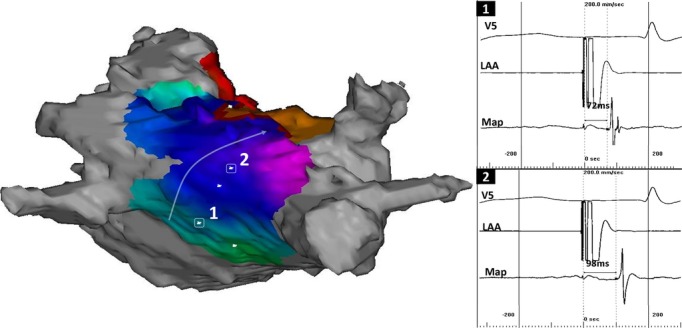
Evaluation of conduction block across the roof during pacing from the LA appendage (LAA). A, Color‐coded 3D activation map showing a caudocranial activation of the posterior wall. B, Electrical activation showing shorter activation times at the lower (1: 72 ms) than at the upper posterior wall (2: 98 ms). LA indicates left atrium.
All procedural endpoints were evaluated in sinus rhythm. Electrical cardioversion was performed when necessary.
Postprocedural Management
After the procedure, a TTE was performed to rule out pericardial effusion. Anticoagulation was started by LMWH and acenocumarol afterward until an international normalized ratio >2 was achieved, then maintained for 3 months in all patients and according to the individual cardioembolic risk score thereafter. Antiarrhythmic treatment was given to all patients during the first 3 months after the procedure: flecainide (100 mg BID) in the absence of structural heart disease and amiodarone (200 mg QD) or sotalol (80 to 160 mg BID), if present. Afterward, antiarrhythmic drugs were interrupted and only reintroduced if any arrhythmic event was documented.
Follow‐up
Follow‐up was performed at the outpatient clinic at 1, 3, and 6 months after the procedure and every 6 months thereafter. Evaluation included assessment of arrhythmia‐related symptoms, adverse events, and treatment adherence and any additional therapy since the previous follow‐up visit. Routine 48‐hour Holter monitoring was performed before each visit, and patients were asked to go to an emergency department if any symptom suggestive of recurrence occurred between scheduled visits. The minimum follow‐up of the study population was 6 months after the AF ablation procedure.
Definitions
Arrhythmia recurrence is defined as any arrhythmia of ≥30 seconds documented by an ECG or device recording system, after a 3‐month blanking period. Cavo‐tricuspid isthmus‐dependent flutter was not considered recurrence.
A blanking period of 3 months was employed after ablation. Recurrences within these first 3 months were not classified as failure of the procedure. Any patient suffering a persistent atrial arrhythmia during this period was reverted to sinus rhythm by electrical cardioversion.
Statistical Analysis
The primary endpoint of the study was freedom from arrhythmia recurrence after a single ablation procedure. Any episode of AF or LA flutter within the first 3 months after the procedure was considered part of the blanking period (not recurrence). The ablation group was blinded to patients and to the physicians evaluating the outcome of the procedure.
On the basis of our group's experience, at 6‐month follow‐up, we expected 55% of patients to be free of arrhythmia after a single PV isolation procedure. With a sample size of 60 patients per arm, a log‐rank test for equality of survival curves will have 80% power and a 2‐sided α‐value of 0.05 to detect an expected 20% reduction in freedom from arrhythmia in the LARA‐1 group. Randomization was performed according to a computer‐generated algorithm in blocks of 20 patients. Inclusion was stopped once 120 patients were enrolled.
Continuous data are presented as the mean value±SD. Qualitative variables are expressed as the number of cases and percentages. To compare means of 2 variables within the same group, we used the paired‐samples Student t test.
Arrhythmia‐free survival curves for each group were compared by log‐rank test and presented as Kaplan‐Meier plots. Cox's method was used to estimate the effect of the LA roof linear ablation after adjusting for baseline variables. The following potential predictors of recurrence were considered: age, sex, duration of AF, LA diameter, LV end‐diastolic, and end‐systolic diameters, LVEF, hypertension, and structural heart disease. The backward step‐wise method with criteria of P<0.10 for inclusion and P>0.10 for removal was used for the final model. A 2‐sided probability value <5% (P<0.05) was considered statistically significant. Statistical analysis was performed using R software (version 3.0.1; the R Project for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics and Procedural Data
One hundred and twenty patients were randomized for analysis: 59 patients in the LARA‐1 group and 61 patients in the LARA‐2 group (Figure 1). The mean age was 55±11, 20% were older than 65 years, and 71% were males. A previous history of stroke was noted in 0.8% of patients and the CHA2DS2‐VASc score was ≥1 in 52.5%. No significant baseline differences were observed between groups (Table 1). Procedural details are provided in Table 2.
Table 1.
Baseline Characteristics
| LARA‐1 (n=59) | LARA‐2 (n=61) | P Value | Total (n=120) | |
|---|---|---|---|---|
| Age, y | 55±11 | 55±12 | 0.857 | 55±11 |
| Males | 42 (71.2%) | 42 (68.9%) | 0.670 | 84 (70.7) |
| Hypertension | 19 (32.2%) | 24 (39.3%) | 0.455 | 43 (35.8%) |
| Diabetes | 2 (3.4%) | 4 (6.6%) | 0.438 | 6 (5%) |
| Previous cardioembolic event | 1 (1.7%) | 0 | 0.987 | 1 (0.8%) |
| Sports practice | 12 (20.3%) | 6 (9.8%) | 0.117 | 18 (15%) |
| Obstructive sleep apnea | 3 (5.1%) | 5 (8.2%) | 0.510 | 8 (6.7%) |
| Lone AF | 45 (76.3%) | 54 (88.5%) | 0.127 | 90 (82.5%) |
| Structural cardiomyopathy | 14 (23.7%) | 7 (11.7%) | 0.200 | 21 (17.5%) |
| Tachycardiomyopathy | 1 (1.7%) | 0 | 1 (0.8%) | |
| Ischemic | 4 (6.8%) | 2 (3.3%) | 6 (5%) | |
| Hypertrophic | 1 (1.7%) | 0 | 1 (0.8%) | |
| Valvular | 6 (10.2%) | 1 (1.6%) | 7 (5.8%) | |
| Hypertensive | 2 (3.4%) | 3 (4.9%) | 5 (4.2%) | |
| Others | 0 | 1 (1.6%) | 1 (0.8%) | |
| CHADS2 ≥1 | 20 (33.9%) | 26 (42.6%) | 0.341 | 46 (38.3%) |
| CHA2DS2‐VASc ≥1 | 31 (52.5%) | 32 (52.4%) | 1 | 63 (52.5%) |
| AF duration, months | 59±59 | 60±56 | 0.902 | 60±57 |
| LA anteroposterior diameter, mm | 41±6 | 41±6 | 0.924 | 41±6 |
| LV ejection fraction, % | 62±7 | 62±5 | 0.774 | 62±6 |
| LV end‐diastolic diameter, mm | 52±4 | 51±5 | 0.108 | 52±5 |
| LV end‐systolic diameter, mm | 33±5 | 32±5 | 0.534 | 33±5 |
AF indicates atrial fibrillation; LA, left atrium; LV, left ventricle.
Table 2.
Procedural Data
| LARA‐1 (n=59) | LARA‐2 (n=61) | P Value | Total (n=120) | |
|---|---|---|---|---|
| Procedural time, minutes | 172±53 | 153±51 | 0.063 | 163±58 |
| Radiofrequency time, minutes | 50±12 | 47±13 | 0.302 | 48±13 |
| Fluoroscopy time, minutes | 32±35 | 22±13 | 0.053 | 27±27 |
| PV isolation | ||||
| Left superior | 59 (100%) | 61 (100%) | 0.285 | 120 (100%) |
| Left inferior | 57 (96.6%) | 57 (93.4%) | 0.416 | 114 (95%) |
| Right superior | 57 (96.6%) | 57 (93.4%) | 0.478 | 118 (98.3) |
| Right inferior | 56 (94.91%) | 61 (100%) | 0.325 | 114 (95%) |
| Overall | 52 (88.5%) | 58 (95.0%) | 0.843 | 106 (89.1%) |
| Complications | 4 (6.7%) | 5 (8.5%) | 0.390 | 9 (7.5%) |
| Stroke | 2 (3.4%) | 1 (1.6%) | 0.97 | 3 (2.5%) |
| Coronary ischemia | 0 | 2 (3.3%) | 0.164 | 2 (1.7%) |
| Pericarditis | 0 | 1 (1.6%) | 0.327 | 1 (0.8%) |
| Cardiac tamponade | 1 (1.7%) | 0 | 0.303 | 1 (0.8%) |
| Esophagitis | 0 | 0 | — | 0 |
| Fever | 1 (1.7%) | 0 | 0.303 | 0 |
| Vascular complications | 0 | 3 (4.9%) | 0.087 | 3 (2.5%) |
| PV stenosis | 0 | 0 | — | 0 |
| Mitral valve rupture | 1 (1.7%) | 0 | — | 1 (0.8%) |
LARA indicates left atrial roof linear ablation; PV, pulmonary vein.
PV isolation was attempted in all patients. Of these, complete bidirectional conduction block was achieved in 89.1% (88.5% in LARA‐1 and 95% in LARA‐2; P=0.843), with no difference in RF duration (50±12 minutes vs. 47±13 minutes; P=0.302) or procedural time (172±53 minutes vs. 153±51 minutes; P=0.063) between LARA‐1 and LARA‐2, respectively. There was a trend to longer fluoroscopy time in group LARA‐1 that did not reach statistical significance (fluoroscopy time [32±35 minutes vs. 22±13 minutes; P=0.053]).
During LA appendage pacing, activation mapping confirmed complete conduction block across the RL in 81.4% of cases in LARA‐1 (Figure 4). Patients with successful RL linear block were significantly older (LARA‐1 without block: 46±13.7 years vs. LARA‐1 with block: 57.1±8.8 years; P=0.025). No other predictor for successful RL block could be identified.
Figure 4.
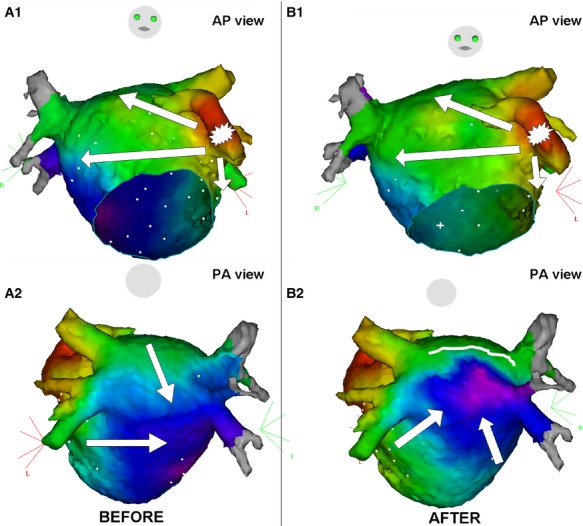
Color‐coded 3D activation map during pacing from the LA appendage in a patient undergoing a first ablation procedure for paroxysmal AF. A, Anteroposterior and posteroanterior views before linear ablation at the LA roof showing 2 activation fronts at the posterior wall. B, Anteroposterior and posteroanterior views after linear ablation at the LA roof showing the caudocranial activation of the LA posterior wall. AF indicates atrial fibrillation; AP, anteroposterior; LA, left atrium; PA, posteroanterior.
Overall, the endpoints of the procedure were confirmed using previously described criteria in 45 and 54 patients of the LARA‐1 (PV isolation and LA roof block) and LARA‐2 (PV isolation) groups, respectively (76.3% vs. 88.5%; P=0.127).
Follow‐up
One LARA‐1 patient was lost to follow‐up because of cancer and subsequent death. Table 3 summarizes the information at follow‐up. After a mean follow‐up of 15±10 months (median, 12), 35 patients in group LARA‐1 (28 with confirmed conduction block across the RL and 7 without complete RL block) and 34 in group LARA‐2 had no documented arrhythmia recurrence after a single ablation procedure. No statistically significant differences were observed in arrhythmia recurrence between those with or without ablation of the LA roof (long‐rank test, P=0.877; Figure 5A).
Table 3.
Follow‐up Data After First Procedure
| LARA‐1 (n=59) | LARA‐2 (n=61) | P Value | Total (n=120) | |
|---|---|---|---|---|
| Follow‐up duration, months | 13±9 | 16±11 | 0.238 | 15±10 |
| Recurrence rate during blanking period | 16 (27.1%) | 17 (27.9%) | 1 | 33 (27.5%) |
| Type of recurrence during blanking period | ||||
| Atrial fibrillation | 16 (27.1%) | 13 (21.3%) | 0.594 | 28 (23.3%) |
| Left atrial flutter | 0 | 4 (6.6%) | 0.136 | 4 (3.3%) |
| Other | 0 | 0 | — | 0 |
| Recurrence rate during follow‐up | ||||
| >3 months after the procedure | 24 (40.7%) | 27 (44.3%) | 0.832 | 51 (42.5%) |
| Complete conduction block across the RL* | 20 (42.6%) | — | — | |
| Incomplete conduction block across the RL* | 4 (36.4%) | — | — | |
| Type of recurrence during follow‐up | ||||
| Atrial fibrillation | 21 (35.6%) | 22 (36.1%) | 1 | 43 (35.8%) |
| Left atrial flutter | 3 (5.1%) | 5 (8.2%) | 0.751 | 8 (6.7%) |
| Other | 0 | 0 | — | 0 |
LARA indicates left atrial roof linear ablation; RL, roof line.
Comparison between patients in group LARA‐1 with complete or incomplete RL conduction block: P=0.655.
Figure 5.
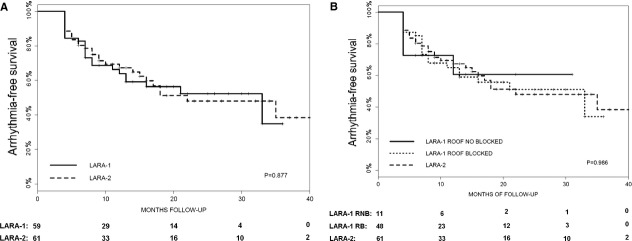
Accumulated arrhythmia‐free survival (Kaplan‐Meier) after a single ablation procedure. A, Comparison between the LARA‐1 (solid line) and LARA‐2 (dotted line) groups. B, Comparison between the LARA‐1 patients that achieved conduction block at the roof (solid line), the LARA‐1 patients that did not achieve conduction block at the roof (slashed line), and the LARA‐2 patients (dotted line). LARA indicates left atrial roof linear ablation.
In the LARA‐1 group, recurrences were the result of AF in 24 patients (40%) and LA flutter in 3 (5.1%). In the LARA‐2 group, 27 patients (45%) had AF recurrences, and 5 (8.2%) had new‐onset LA flutter. Univariate analysis identified AF duration (hazard ratio [HR], 1.01 [95% confidence interval {CI}, 1.002 to 1.012]; P<0.01), as the covariate associated with arrhythmia recurrence (Table 4).
Table 4.
Predictors of All‐Cause Recurrence Risk, Uni‐ and Multivariate Cox Proportional Hazards Models
| No Recurrence (n=69) | Recurrence (n=51) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| Age, y | 54±12 | 57±10 | 1.03 (1.00 to 1.06) | 0.055 | ||
| Males | 50 (72%) | 34 (67%) | 0.84 (0.47 to 1.52) | 0.842 | ||
| Hypertension | 23 (33%) | 20 (39%) | 1.38 (0.78 to 2.54) | 0.266 | ||
| Atrial fibrillation duration, months | 48±39 | 74±72 | 1.01 (1.00 to 1.01) | 0.007 | 1.01 (1.002 to 1.012) | 0.007 |
| Sleep apnea syndrome | 3 (4%) | 5 (10%) | 2.46 (0.97 to 6.28) | 0.059 | ||
| Athlete | 8 (12%) | 6 (12%) | 0.88 (0.37 to 2.10) | 0.771 | ||
| Cardiomyopathy | 12 (17%) | 9 (18%) | 0.98 (0.48 to 2.00) | 0.948 | ||
| LVEF (per % increase) | 62±7 | 62±5 | 1.00 (0.96 to 1.05) | 0.961 | ||
| LA diameter | 41±5 | 41±6 | 1.01 (0.96 to 1.07) | 0.599 | ||
| CHADS2 score | 3 (4%) | 2 (4%) | 1.23 (0.30 to 5.09) | 0.777 | ||
| CHA2DS2‐VASc score | 31 (45%) | 29 (57%) | 1.48 (0.84 to 2.62) | 0.178 | ||
| LARA‐1 (roof line) | 34 (49%) | 24 (47%) | 1.04 (0.60 to 1.81) | 0.880 | ||
CI indicates confidence interval; HR, hazard ratio; LA, left atrium; LARA, left atrial roof linear ablation; LVEF, left ventricular ejection fraction.
The 12‐month results (Figure 5B) were not influenced by achievement of complete block across the RL in LARA‐1 patients (HR, 0.96 [95% CI, 0.55 to 1.67]; P=0.880) or by complete bidirectional block of all PV (HR, 0.986 [95% CI, 0.806 to 1.207]; P=0.843).
Complications
In the LARA‐1 group, 1 patient experienced cardiac tamponade, which was successfully drained percutaneously, requiring no further intervention; another patient had a transient cerebrovascular ischemic attack, resolved by unfractionated heparin with no documented lesion on the CT scan and no sequelae. Two patients in the LARA‐2 group had transient inferior myocardial ischemia, probably related to air embolism during transseptal catheterization, which was resolved with intravenous nitroglycerin (100 μg) within a few minutes; additionally, 1 patient had postprocedural pericarditis without significant pericardial effusion that required nonsteroidal anti‐inflammatory therapy for 1 week. Finally, there was 1 mitral valve rupture in the LARA‐1 group, secondary to entrapment of the circular catheter among the chords; the patient had to undergo emergent mitral valve repair. No symptomatic stenosis of the PV was documented by MR at 12 months. There were no significant differences in complication occurrence between groups (Table 2).
Second Ablation Procedures
The ablation procedure was repeated 15.7±11 months after the index procedure in 27 (22%) patients (14 in LARA‐1 and 13 in LARA‐2; P=0.92) because of new‐onset LA flutter in 6 patients (4 in LARA‐1 and 2 in LARA‐2; P=0.64) and AF recurrence in the remaining 21 patients. Overall, the 120 patients included in the study underwent 1.41±0.57 AF ablation procedures (1.49±0.646 in LARA‐1 and 1.34±0.476 in LARA‐2; P=0.114).
Recovery of PV conduction was observed in a mean of 3.48±0.749 PV per patient, while no patient had all PVs isolated. The identified conduction gaps were located at the left superior PV in 17 patients (62.9%), left inferior PV in 19 (70.3%), right superior PV in 20 (74.1%), and right inferior PV in 17 (62.9%). Recovery of conduction across the LA roof line was observed in 6 patients (42.8%). All 6 patients with LA flutter showed gaps at the level of the PV responsible for the reentrant arrhythmia. There were no macroreentries across the LA roof or mitral‐isthmus–dependent flutter. The ablation strategy of the repeat procedures consisted in PV isolation for all patients and ablation along the RL to close the gap in those patients from group LARA‐1 with recovered conduction (Figure 6).
Figure 6.
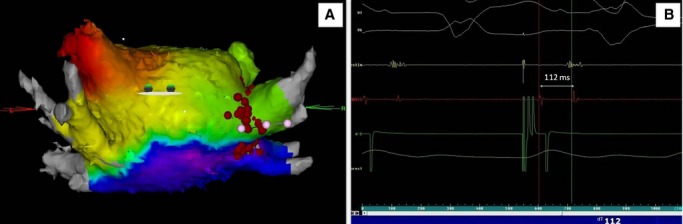
Confirmation of persistent conduction block at the LA roof in a patient undergoing a repeat ablation procedure for recurrent paroxysmal AF. A, Color‐coded 3D activation map showing a caudocranial activation of the posterior wall. B, Double potential at the roof line (112 ms between spikes). AF indicates atrial fibrillation; LA, left atrium.
After a follow‐up of 15.3±10.2 months after the last ablation procedure, 70.8% of the patients of this series remained arrhythmia free with no difference in arrhythmia‐free survival between groups (Table 5). Thirty‐one patients had AF recurrence (14 in group LARA‐1 and 17 in LARA‐2; P=0.079).
Table 5.
Follow‐up Data After Last Procedure
| LARA‐1 (n=59) | LARA‐2 (n=61) | P Value | Total (n=120) | |
|---|---|---|---|---|
| Follow‐up duration, months | 14.06±8.764 | 16.39±11.33 | 0.230 | 15.25±10.17 |
| Recurrence rate during follow‐up | ||||
| >3 months after the procedure | 15 (25.4%) | 19 (31.1%) | 0.622 | 34 (28.3%) |
| Type of recurrence during follow‐up | ||||
| Atrial fibrillation | 14 (23.7%) | 17 (27.9%) | 0.755 | 31 (25.8%) |
| Left atrial flutter | 1 (0.02%) | 3 (0.05%) | 0.635 | 4 (0.03%) |
| Other | 0 | 0 | — | 0 |
LARA indicates left atrial roof linear ablation.
Discussion
The main finding of this prospective, randomized study is that linear ablation at the LA roof does not improve 12‐month success after PV isolation for paroxysmal AF, independently of the achievement of bidirectional block. Furthermore, the incidence of macroreentrant arrhythmias after a single ablation in this type of patient did not differ significantly between groups.
Linear Ablation for AF Ablation
Currently, circumferential PV ablation with verification of electrical disconnection, associated or not to additional linear lesions, probably represents the most accepted ablation strategy in PAF.1–4 However, despite the various ablation strategies available, single‐procedure efficacy is still unsatisfactory: 60% to 80% in PAF, with 40% to 50% of patients requiring a second procedure to achieve long‐term freedom from AF.16–18 Because the efficacy of AF ablation seems to be related to the extent of ablated tissue,19 there has been a trend toward more‐extensive ablation. The creation of additional lesions on top of the isolation of the PV has been proposed in an attempt to obtain success comparable to that obtained with surgical linear ablation lesions. Still, the existing evidence provides contradictory information about the usefulness, number, and location of linear lesions.
The role of additional lines in cases of PAF is particularly unclear. It has been suggested that ablation lines along the roof of the LA and mitral isthmus may improve clinical outcomes in PAF.7,9,20 Conversely, the exclusion of the LA posterior wall has no effect on the incidence of AF recurrences after circumferential PV ablation, in a randomized trial of 120 patients (60% in PAF).13 Finally, a randomized, prospective trial comparing segmental PV isolation and circumferential PV ablation plus linear ablation at the LA roof and MI showed that significantly more patients had LA flutter in the linear ablation group.21
To our knowledge, only 2 other randomized studies have evaluated the effect of linear ablation at the LA roof, with conflicting results.9,22 Hocini et al.9 randomized 90 patients to PV isolation with or without LA roof linear ablation (1:1 ratio). In their study, additional roofline ablation was associated with 87% of patients being arrhythmia free without antiarrhythmics, compared with 69% undergoing PV isolation alone, after a follow‐up of 15±4 months.
More recently, Mun et al.22 randomized 156 patients into 3 different groups (1:1:1 ratio): PV isolation alone, PV isolation together with LARA, and PV isolation with the “box lesion” (RL+posterior line); the findings did not confirm those described by Hocini et al.,9 because the addition of the LA roofline did not improve the clinical outcome at 15±6 months after the index procedure.
In our randomized study, the deployment of an ablation line at the LA roof did not improve the arrhythmia recurrence rate. The reason for the disparate findings may be related to differences in PV isolation techniques, rather than to the LARA itself. Wider antral PV isolation is likely to reduce the critical mass for maintenance of AF more than the more ostial approach performed by Hocini et al.9,23
Linear Ablation to Prevent LA Macroreentrant Arrhythmias
Linear ablation in addition to PV isolation was initially suggested as a means to prevent atrial tachycardia after circumferential PV ablation.8 However, previous results from our group13 and others21 have not shown any benefit from additional linear lesions in terms of the risk of LA flutter. The present study also supports the idea that additional ablation lines may not be necessary in patients with PAF to prevent post‐AF ablation macroreentrant arrhythmias. This is most probably because the predominant underlying mechanism of AF in these patients is mainly focal triggers; the substrate does not play a relevant role. Favoring this theory, all second procedures to treat LA flutter after the index ablation showed a reentrant circuit using gaps located at the previous PV encircling lesions or at the LARA, in accord with other series.13,24 The difficulty in achieving continuity around ipsilateral PVs has already been described, especially at the septal aspect and the area between the LA appendage and left superior PV.25 Furthermore, it has been widely shown that macroreentrant arrhythmias during follow‐up are frequently related to gaps in previous linear lesions.6,10,12,21,24,26–29 Therefore, if additional linear lesions are applied, line completeness should be demonstrated by mapping or pacing maneuvers.
Clinical Implications
The results of the study clearly show that ablation of the LA roof in addition to PV isolation does not add clinical benefit, compared with PV isolation alone in patients with PAF. Although the total procedural time and RF delivery duration did not increase significantly when the ablation line was added, care must be taken to avoid potential risks, such as perforation or esophageal damage. There was also a trend to longer fluoroscopy time in the LARA‐1 group, although it did not reach statistical significance, probably because of lack of statistical power.
On the other hand, linear lesions may promote LA macroreentrant arrhythmias secondary to conduction recovery along the line. Therefore, the results of this randomized study further support the thesis that linear ablation at the LA roof is not necessary as a predefined lesion set to treat PAF.
Study Limitations
The study was planned to detect an absolute reduction of 20% in the proportion of arrhythmia‐free patients assuming that 55% of patients would be recurrence free at 6 months, based on the earlier experience of our group. Yet, the recurrence‐free survival at 6 months was closer to 80. This could reduce the power to detect differences among groups within the study. However, the same number of patients were arrhythmia free in both groups; consequently, the formal comparison is far from the 5% significance level and the adjusted HR for arrhythmia recurrence was close to 1. Therefore, the lack of statistical significance may not be attributable to low statistical power. On the other hand, the lack of the sample size was not calculated to take into account possible subgroup analyses; therefore, the absence of identifiable predictors of successful RL block could be secondary to lack of statistical power.
The study cannot exclude that the LA roof linear ablation may have some effect in individual AF cases because the effect of this ablation was evaluated as part of a predefined lesion set performed in all patients. Tailored approaches for AF ablation have been proposed.30–32 Finally, arrhythmia recurrence was detected by routine 48‐hour Holter monitoring and ECG recording when symptoms occurred between scheduled visits. It is likely that some arrhythmia recurrences may have escaped detection in asymptomatic patients. However, this limitation affects both ablation groups equally and therefore should not affect the study conclusions. Moreover, this pragmatic approach has been taken in many other published studies.7,9,11,33–36
Conclusion
This double‐blinded, prospective, randomized study shows that there is not sufficient evidence to suggest that linear ablation at the roof of the LA improves the mid‐term results of PV isolation for the treatment of PFA, independently of the achievement of bidirectional block.
Disclosures
Dr Elena Arbelo discloses lecture horonariums by Biosense Webster. Dr José María Tolosana discloses research funding, lecture honorariums, and consulting for St. Jude Medical. Dr Antonio Berruezo discloses research funding, lecture honorariums, and consulting for Biosense Webster and St. Jude Medical. Prof Josep Brugada discloses research funding, lecture honorariums, and consulting for Biosense Webster and St. Jude Medical. Dr Lluís Mont discloses research funding, lecture honorariums, and consulting for Biosense Webster and St. Jude Medical. Dr Elena Arbelo discloses lecture horonariums by Biosense Webster. There is no conflict of interest to disclose for the remaining authors.
Acknowledgments
The authors thank Elaine Lilly, PhD, for manuscript editing and Neus Portella, RS, for administrative assistance.
References
- 1.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005; 111:1100-1105. [DOI] [PubMed] [Google Scholar]
- 2.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010; 3:32-38. [DOI] [PubMed] [Google Scholar]
- 3.Arbelo E, Brugada J, Hindricks G, Maggioni A, Tavazzi L, Vardas P, Anselme F, Inama G, Jais P, Kalarus Z, Kautzner J, Lewalter T, Mairesse G, Perez‐Villacastin J, Riahi S, Taborsky M, Theodorakis G, Trines S. ESC‐EURObservational Research Programme: the Atrial Fibrillation Ablation Pilot Study, conducted by the European Heart Rhythm Association. Europace. 2012; 14:1094-1103. [DOI] [PubMed] [Google Scholar]
- 4.Arbelo E, Brugada J, Hindricks G, Maggioni AP, Tavazzi L, Vardas P, Laroche C, Anselme F, Inama G, Jais P, Kalarus Z, Kautzner J, Lewalter T, Mairesse GH, Perez‐Villacastin J, Riahi S, Taborsky M, Theodorakis G, Trines SAon the behalf of the Atrial Fibrillation Ablation Pilot Study Investigators. The atrial fibrillation ablation pilot study: an European Survey on Methodology and results of catheter ablation for atrial fibrillation: conducted by the European Heart Rhythm Association. Eur Heart J. 2014; 35:1466-1478. [DOI] [PubMed] [Google Scholar]
- 5.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJE, Rao SN, DiBella EVR, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed‐enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009; 119:1758-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ernst S, Ouyang F, Lober F, Antz M, Kuck KH. Catheter‐induced linear lesions in the left atrium in patients with atrial fibrillation: an electroanatomic study. J Am Coll Cardiol. 2003; 42:1271-1282. [DOI] [PubMed] [Google Scholar]
- 7.Jais P, Hocini M, Hsu LF, Sanders P, Scavee C, Weerasooriya R, Macle L, Raybaud F, Garrigue S, Shah DC, Le Metayer P, Clementy J, Haissaguerre M. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004; 110:2996-3002. [DOI] [PubMed] [Google Scholar]
- 8.Pappone C, Manguso F, Vicedomini G, Gugliotta F, Santinelli O, Ferro A, Gulletta S, Sala S, Sora N, Paglino G, Augello G, Agricola E, Zangrillo A, Alfieri O, Santinelli V. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation. 2004; 110:3036-3042. [DOI] [PubMed] [Google Scholar]
- 9.Hocini M, Jais P, Sanders P, Takahashi Y, Rotter M, Rostock T, Hsu LF, Sacher F, Reuter S, Clementy J, Haissaguerre M. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation. 2005; 112:3688-3696. [DOI] [PubMed] [Google Scholar]
- 10.Willems S, Klemm H, Rostock T, Brandstrup B, Ventura R, Steven D, Risius T, Lutomsky B, Meinertz T. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison. Eur Heart J. 2006; 27:2871-2878. [DOI] [PubMed] [Google Scholar]
- 11.Sanders P, Hocini M, Jaïs P, Sacher Fd, Hsu L‐F, Takahashi Y, Rotter M, Rostock T, Nalliah CJ, Clémenty J, Haïssaguerre M. Complete isolation of the pulmonary veins and posterior left atrium in chronic atrial fibrillation. Long‐term clinical outcome. Eur Heart J. 2007; 28:1862-1871. [DOI] [PubMed] [Google Scholar]
- 12.Knecht S, Hocini M, Wright M, Lellouche N, O'Neill MD, Matsuo S, Nault I, Chauhan VS, Makati KJ, Bevilacqua M, Lim K‐T, Sacher F, Deplagne A, Derval N, Bordachar P, Jais P, Clementy J, Haissaguerre M. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J. 2008; 29:2359-2366. [DOI] [PubMed] [Google Scholar]
- 13.Tamborero D, Mont L, Berruezo A, Matiello M, Benito B, Sitges M, Vidal B, de Caralt TM, Perea RJ, Vatasescu R, Brugada J. Left atrial posterior wall isolation does not improve the outcome of circumferential pulmonary vein ablation for atrial fibrillation: a prospective randomized study. Circ Arrhythm Electrophysiol. 2009; 2:35-40. [DOI] [PubMed] [Google Scholar]
- 14.Calvo N, Mont L, Vidal B, Nadal M, Montserrat S, Andreu D, Tamborero D, Pare C, Azqueta M, Berruezo A, Brugada J, Sitges M. Usefulness of transoesophageal echocardiography before circumferential pulmonary vein ablation in patients with atrial fibrillation: is it really mandatory? Europace. 2011; 13:486-491. [DOI] [PubMed] [Google Scholar]
- 15.Sang C, Jiang C, Dong J, Liu X, Yu R, Long D, Tang R, Gao L, Ning M, Chen G, Li W, Ma C. A new method to evaluate linear block at the left atrial roof: is it reliable without pacing? J Cardiovasc Electrophysiol. 2010; 21:741-746. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang F, Tilz R, Chun J, Schmidt B, Wissner E, Zerm T, Neven K, Kokturk B, Konstantinidou M, Metzner A, Fuernkranz A, Kuck KH. Long‐term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5‐year follow‐up. Circulation. 2010; 122:2368-2377. [DOI] [PubMed] [Google Scholar]
- 17.Hussein AA, Saliba WI, Martin DO, Bhargava M, Sherman M, Magnelli‐Reyes C, Chamsi‐Pasha M, John S, Williams‐Adrews M, Baranowski B, Dresing T, Callahan T, Kanj M, Tchou P, Lindsay BD, Natale A, Wazni O. Natural history and long‐term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol. 2011; 4:271-278. [DOI] [PubMed] [Google Scholar]
- 18.Medi C, Sparks PB, Morton JB, Kistler PM, Halloran K, Rosso R, Vohra JK, Kumar S, Kalman JM. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long‐term follow‐up. J Cardiovasc Electrophysiol. 2011; 22:137-141. [DOI] [PubMed] [Google Scholar]
- 19.Katritsis D, Ellenbogen K, Giazitzoglou E, Sougiannis D, Paxinos G, Fragakis N, Camm A. Clinical outcome of left atrial ablation for paroxysmal atrial fibrillation is related to the extent of radiofrequency ablation. J Interv Card Electrophysiol. 2008; 22:31-37. [DOI] [PubMed] [Google Scholar]
- 20.Gaita F, Caponi D, Scaglione M, Montefusco A, Corleto A, Di Monte F, Coin D, Di Donna P, Giustetto C. Long‐term clinical results of 2 different ablation strategies in patients with paroxysmal and persistent atrial fibrillation/clinical perspective. Circ Arrhythm Electrophysiol. 2008; 1:269-275. [DOI] [PubMed] [Google Scholar]
- 21.Sawhney N, Anousheh R, Chen W, Feld GK. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2010; 3:243-248. [DOI] [PubMed] [Google Scholar]
- 22.Mun H‐S, Joung B, Shim J, Hwang HJ, Kim JY, Lee M‐H, Pak HN. Does additional linear ablation after circumferential pulmonary vein isolation improve clinical outcome in patients with paroxysmal atrial fibrillation? Prospective randomised study. Heart. 2012; 98:480-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haissaguerre M, Sanders P, Hocini M, Hsu LF, Shah DC, Scavee C, Takahashi Y, Rotter M, Pasquie JL, Garrigue S, Clementy J, Jais P. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004; 109:3007-3013. [DOI] [PubMed] [Google Scholar]
- 24.Chae S, Oral H, Good E, Dey S, Wimmer A, Crawford T, Wells D, Sarrazin JF, Chalfoun N, Kuhne M, Fortino J, Huether E, Lemerand T, Pelosi F, Bogun F, Morady F, Chugh A. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol. 2007; 50:1781-1787. [DOI] [PubMed] [Google Scholar]
- 25.Mesas CE, Augello G, Edward Lang CC, Gugliotta F, Vicedomini G, Sora N, De Paola AAV, Pappone C. Electroanatomic remodeling of the left atrium in patients undergoing repeat pulmonary vein ablation: mechanistic insights and implications for ablation. J Cardiovasc Electrophysiol. 2006; 17:1279-1285. [DOI] [PubMed] [Google Scholar]
- 26.Chugh A, Oral H, Lemola K, Hall B, Cheung P, Good E, Tamirisa K, Han J, Bogun F, Pelosi F, Morady F. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm. 2005; 2:464-471. [DOI] [PubMed] [Google Scholar]
- 27.Haissaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, Takahashi Y, Rostock T, Hsu LF, Bordachar P, Reuter S, Roudaut R, Clementy J, Jais P. Catheter ablation of long‐lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005; 16:1138-1147. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo S, Wright M, Knecht S, Nault I, Lellouche N, Lim KT, Arantes L, O'Neill MD, Hocini M, Jais P, Haissaguerre M. Peri‐mitral atrial flutter in patients with atrial fibrillation ablation. Heart Rhythm. 2010; 7:2-8. [DOI] [PubMed] [Google Scholar]
- 29.Ouyang F, Ernst S, Vogtmann T, Goya M, Volkmer M, Schaumann A, Bansch D, Antz M, Kuck KH. Characterization of reentrant circuits in left atrial macroreentrant tachycardia: critical isthmus block can prevent atrial tachycardia recurrence. Circulation. 2002; 105:1934-1942. [DOI] [PubMed] [Google Scholar]
- 30.Jais P, Hocini M, Sanders P, Hsu LF, Takahashi Y, Rotter M, Rostock T, Sacher F, Clementy J, Haissaguerre M. Long‐term evaluation of atrial fibrillation ablation guided by noninducibility. Heart Rhythm. 2006; 3:140-145. [DOI] [PubMed] [Google Scholar]
- 31.Oral H, Chugh A, Good E, Sankaran S, Reich SS, Igic P, Elmouchi D, Tschopp D, Crawford T, Dey S, Wimmer A, Lemola K, Jongnarangsin K, Bogun F, Pelosi F, Jr, Morady F. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation. 2006; 113:1824-1831. [DOI] [PubMed] [Google Scholar]
- 32.Tamborero D, Mont L, Molina I, Matiello M, Berruezo A, Sitges M, Perea RJ, de Caralt TM, Vidal B, Zamorano N, Brugada J. Selective segmental ostial ablation and circumferential pulmonary veins ablation. Results of an individualized strategy to cure refractory atrial fibrillation. J Interv Card Electrophysiol. 2007; 19:19-27. [DOI] [PubMed] [Google Scholar]
- 33.Lemola K, Oral H, Chugh A, Hall B, Cheung P, Han J, Tamirisa K, Good E, Bogun F, Pelosi F, Jr, Morady F. Pulmonary vein isolation as an end point for left atrial circumferential ablation of atrial fibrillation. J Am Coll Cardiol. 2005; 46:1060-1066. [DOI] [PubMed] [Google Scholar]
- 34.Karch MR, Zrenner B, Deisenhofer I, Schreieck J, Ndrepepa G, Dong J, Lamprecht K, Barthel P, Luciani E, Schomig A, Schmitt C. Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: a randomized comparison between 2 current ablation strategies. Circulation. 2005; 111:2875-2880. [DOI] [PubMed] [Google Scholar]
- 35.Calo L, Lamberti F, Loricchio ML, De Ruvo E, Colivicchi F, Bianconi L, Pandozi C, Santini M. Left atrial ablation versus biatrial ablation for persistent and permanent atrial fibrillation: a prospective and randomized study. J Am Coll Cardiol. 2006; 47:2504-2512. [DOI] [PubMed] [Google Scholar]
- 36.Arentz T, Weber R, Burkle G, Herrera C, Blum T, Stockinger J, Minners J, Neumann FJ, Kalusche D. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. 2007; 115:3057-3063. [DOI] [PubMed] [Google Scholar]


