Abstract
Background:
In 2003 the outbreak of highly pathogenic H7 avian influenza occurred in the Netherlands. The avian H7 virus causing the outbreak was also detected in humans; one person died of pneumonia and acute respiratory distress syndrome. Our paper describes preclinical studies of a H7N3 live attenuated influenza A vaccine (LAIV) candidate in various animal models.
Objectives:
To study safety, immunogenicity and protection of H7N3 LAIV candidate in mice, ferrets and chickens.
Methods:
The vaccine was generated by a classical reassortment between low pathogenicity A/mallard/Netherlands/00 (H7N3) virus and A/Leningrad4/17/57 (H2N2) master donor virus (MDV).
Results:
Immunogenicity was found that H7N3 LAIV was similar to the MDV in terms of replication in the respiratory organs of mice and failed to replicate in mouse brains. One dose of a H7N3 LAIV elicited measurable antibody response and it was further boosted with a second vaccine dose. Immunization of mice with H7N3 LAIV provided protection against infection following a homologous challenge with wild type H7N3 virus. Attenuated phenotype of H7N3 LAIV has been confirmed in ferrets. Immunogenicity and protective efficacy of H7N3 LAIV in ferrets were also demonstrated. The vaccine protected animals from subsequent infection with wild type H7N3 virus. The results of histopathology study revealed that inoculation of H7N3 LAIV in ferrets did not cause any inflammation or destructive changes in lungs.
Lack of H7N3 LAIV replication in chicken demonstrated complete safety of this preparation for poultry.
Conclusion:
Results of our study suggest that new H7N3 LAIV candidate is safe, immunogenic and protects from homologues influenza virus infection in mice and ferrets.
Keywords: H7N3, influenza, live attenuated influenza vaccine, preclinical studies, pre-pandemic vaccine.
INTRODUCTION
The recent emergence of a new pandemic H1N1 influenza virus (the genus Influenzavirus, the family Orthomyxoviridae) and the threat of transmission of avian viruses to humans had stimulated research and development of live attenuated cold-adapted (ca) influenza vaccines (LAIV) against newly appeared influenza viruses. The World Health Organization (WHO) recommends the development and stockpiling of influenza vaccines for all potential pandemic strains. Several preparations of LAIV against pandemic influenza strains, including H1N1, H5N1, H9N2 and H7N3 have recently been discovered in preclinical and Phase I clinical studies [1].
This paper describes the preclinical studies of a new H7N3 LAIV candidate in mice, ferrets and chickens. In the present study we evaluated a 6:2 reassortant derived from A/Leningrad/134/17/57(H2N2) master donor virus (MDV) and low-pathogenic avian A/mallard/Netherlands/12/00 (H7N3) virus (H7N3-wt). The low pathogenic H7N3-wt avian influenza virus was chosen as a source of the HA and NA gene segments because it had 99% homology in hemagglutinin amino acid sequence with A/Netherlands/ 2003(H7N7) influenza viruses excluding a multibasic cleavage site. The highly pathogenic avian H7N7 virus caused the outbreak in 2003 was detected in 88 humans who suffered from conjunctivitis or mild respiratory symptoms and in one person who died of pneumonia and acute respiratory distress syndrome [2]. According to the hemagglutination inhibition (HI) test with a panel of ferret antisera against different avian and human H7 viruses the H7N3-wt virus was very close to the human H7N7 isolates including fatal case [3]. The HA nucleotide sequence as well as the antigenic profile of the H7N3 LAIV reassortant virus was identical to that of the H7N3-wt parental strain (data not shown), thus demonstrated neither molecular nor antigenic changes had occurred during the reassortment and selection procedure.
The study has addressed the following three objectives: (i). to demonstrate that cold-adapted (ca) reassortant influenza A(H7N3) vaccine candidate is indistinguishable from the parental A/Leningrad/134/17/57 (H2N2) master donor virus (MDV) in regards to replication efficiency in upper and lower respiratory tract of animals; (ii) to evaluate the immunogenicity of different doses of A(H7N3) LAIV vaccine in various animal models; (iii) to study the protective efficacy of cold-adapted reassortant influenza A(H7N3) vaccine candidate in mice and ferrets against a homologous wild type virus challenge.
MATERIALS AND METHODOLOGY
Viruses. The A/17/mallard/Netherlands/00/95 (H7N3) (H7N3 LAIV) reassortant strain that possesses the HA and NA genes from A/mallard/Netherlands/00 (H7N3) wild type virus and the six internal and non-structural protein genes from MDV were prepared by classical genetic reassortment in embryonated chicken eggs (CE) as previously described [4]. The A/mallard/Netherlands/2000 (H7N3) wild type virus was kindly provided by Dr. A.Klimov at the Centers for Disease Control and Prevention (Atlanta, GA, USA). Genome composition of the reassortant influenza virus was assessed by hemagglutination inhibition test (HI) and restriction fragment length polymorphism (RFLP) analysis of viral genes amplified by RT-PCR [5]. The A/Leningrad/ 134/17/57 (H2N2) master donor virus (MDV) and A/17/California/2009/38 (H1N1) LAIV reassortant virus [6] were used as controls in virus replication kinetics and neuroinvasion study in mice and were provided by the Department of Virology, Institute of Experimental Medicine (St.Petersburg, Russia). Viruses were propagated in 10-days old CE for 48 hours at 34°C. The 50% eggs infectious dose (EID50) was determined according to Reed-Muench method [7] and expressed as the mean log10 EID50/mL.
Viral replication kinetics and neuroinvasion in mice. The female Balb/c mice 6 to 8 weeks of age were used for all experiments. For determining infectivity (50% mouse infectious dose, MID50) mice were lightly anesthetized with ether and 50 μL of 101 to 107 EID50 of A/17/mallard/ Netherlands/00/95 (H7N3) or A/Leningrad/134/17/57 (H2N2) MDV diluted in phosphate-buffered saline (PBS) was inoculated intranasally (i.n.) divided equally per nostril. Viral loads were measured in respiratory and brain tissues collected at day 3 and 6 post-infection (dpi). Tissue homogenates were prepared using a disruptor and clarified supernatants were titrated in eggs at permissive temperature to determine infectious virus [8].
Immunogenicity and protection studies in mice. Groups of animals (20 in group) were inoculated with 1000 MID50 (~7 log10 EID50) or 100 MID50 (~6 log10 EID50) of H7N3 LAIV candidate intranasally, after collecting a pre-immunization blood samples. Control mice received intranasally 50 μL of PBS. A second blood sample was collected at 28 dpi. On the same day, the animals received a second intranasal inoculation with the same virus that was used for priming at 0 dpi. To assess protection, all animals at 42 dpi were infected with 100 MID50 (~6 log10 EID50) of A/mallard/Netherlands/12/2000 (H7N3) wild type virus by the intranasal route. Three animals from each group were euthanized at 45 dpi, and the respiratory and systemic organs were harvested for virus titration. HI antibody titers were determined in five individual serum samples collected on days 0, 28 and 42. Body weights were taken daily following challenge through day 14 post-challenge. Sera were tested for HI against homologous H7N3 virus using 0.75% chicken red blood cells [8].
Safety studies on chickens. Gallus domesticus - White Leghorn SPF, 5 weeks of age were used in all experiments. Groups of male and female chickens received intravenous administration of 0.2 mL virus-containing allantoic fluid of H7N3 LAIV (infectious activity 8.1 log10 EID50/0.2 mL) or intranasally 0.1 mL virus-containing allantoic fluid of H7N3 LAIV (infectious activity 6.0 log10 EID50/0.1 mL). On 3 dpi samples of oral and cloacal swabs, tissues of lungs, kidney, heart and brain were collected. Swab samples were inoculated for viral reproduction into 10 day old chicken embryos in 0.2 mL, 3 CE per sample. Tissue samples homogenates were inoculated for viral reproduction into 10-days old chicken embryos in 0.2 mL in dilution 1/10, 3 CE per sample. Blood samples were taken on day 14th after intranasal administration [9].
Safety and attenuated phenotype in ferrets . Male ferrets 4-6 months of age were used for all experiments. All animals were tested prior to the experiment to make sure that they were seronegative to circulating influenza A and B viruses. Groups of six ferrets received once vaccine dose of H7N3 LAIV intranasally of either 6.0 log10 EID50 or 7.0 log10 EID50 in 0.5 mL. The A/mallard/Netherlands/12/00 (H7N3) and A/17/California/2009/38 (H1N1) viruses in dose 7.0 log10 EID50 were used as positive and negative controls respectively. Control group received sterile PBS intranasally. Three ferrets from each group were euthanized on the day three after the beginning of the experiment, and three remaining animals were euthanized on the day five. Virus replication was measured in lungs and nasal turbinates of all sacrificed animals. Detailed observations (including activity, sneezing, lethargic stupor, diarrhea, neurological symptoms etc.) were conducted one week prior to inoculation and daily after immunization. Body temperature and weight were measured in all animals prior to inoculation and daily after immunization.
Immunogenicity and protection study in ferrets. Groups of six ferrets received two administration of 107 EID50 H7N3 LAIV intranasally in the volume 0.5 mL 28 days apart (Day 0 and D28). On day 42 the animals were challenged intranasally with 106 EID50 of wild type influenza virus A/mallard/Netherlands/12/2000 (H7N3) in volume of 0.1 mL. Control animals received 0.1 mL of physiological saline intranasally. Three animals from each group were euthanized on the day three after experimental challenge, and three remaining ferrets euthanized on the day 14. Body weight of the animals measured a week prior to experimental inoculation and on daily basis in the period of 14 days after experimental inoculation. Detailed observations (including activity, sneezing, lethargic stupor, diarrhea, neurologic symptoms, etc) were conducted one week prior to inoculation and on a daily basis after challenge. Temperature was measured in all animals prior to inoculation and on day 0, 1, 2, 7, 28, 30, 35, 42 (i.e. on day 0, 1, 2, and 7 after each vaccine dose). Virus replication was studied in lungs and nasal turbinates of all sacrificed animals. One lung from each sacrificed animal was used for histological analysis, while another lung was used for studying of viral reproduction by plaque assay in MDCK cells. Blood samples were taken prior to the experiment, and on 28th and 42nd day after the beginning of the experiment. Serum samples were stored at -80(C prior to testing. HAI titers were measured for all serum samples, collected on day 0, 28 and 42 by standard procedure [10]. Nasal wash samples were collected on days 3, 5, 7, 9 after challenge and stored -80(C before analysis for viral load.
Statistical analysis. Data were analyzed with «Statistica» software, version 6.0 (StatSoft, Inc. Tulsa, Oklahoma, USA). Comparisons of two independent groups were made with nonparametric Mann-Whitney test. Comparisons of two dependent variables were performed using Wilcoxon matched pairs test. The p-value < 0.05 was considered to be statistically significant.
RESULTS
Vaccine Replication Kinetics and Neuroinvasion in Mice
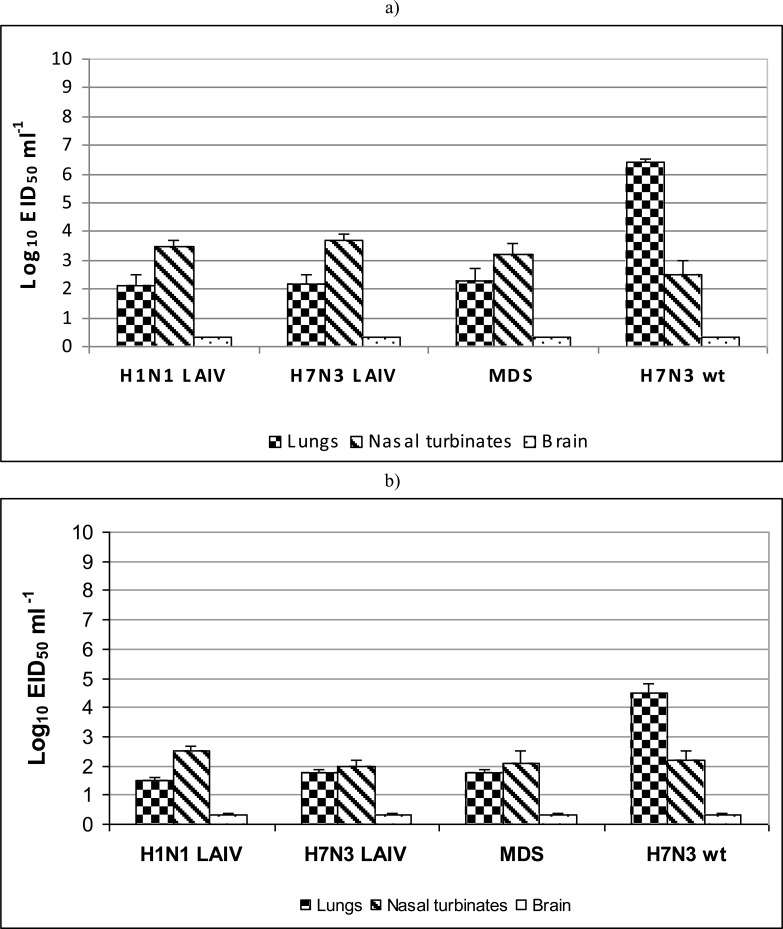
The H1N1 LAIV, H7N3 LAIV and H2N2 MDV influenza viruses at infectious dose of 6 log10 EID50 replicated in mouse lungs at the level 2.1-2.3 log10 EID50/mL at 3 dpi (Fig. 1a). At 6 dpi, replication of the viruses in the lungs decreased to 1.6-2.0 log10 EID50/mL (Fig. 1b). In contrast, the wild type virus A/mallard/Netherlands/12/2000 (H7N3) demonstrated 104 higher level of replication in the lungs on day 3 p.i. (6.2 log10 EID50/mL). The level of replication of studied viruses in nasal turbinates was 2.5-3.7 log10 EID50/mL at 3 dpi (Fig. 1a), and 2.0-2.2 log10 EID50/mL at 6 dpi (Fig. 1b). There was no significant difference between tested LAIV viruses and MDV in regard to replication in upper respiratory tract of mice. It was shown that A/17/mallard/Netherlands/00/95 (H7N3) was indistin-guishable from the MDV and A/17/California/2009/38 (H1N1) vaccine candidate in terms of replication in the lungs and upper respiratory tract of mice at 3 and 6 dpi. No virus was found in the brain tissue of immunized mice at 3 and 6 dpi (in undiluted samples tested). Thus, it was demonstrated that A/17/mallard/Netherlands/00/95 (H7N3) LAIV vaccine candidate is identical to A/Leningrad/134/17/57 (H2N2) MDV and A/17/California/2009/38 (H1N1) vaccine in lacking neuroivasive capacity and that all three viruses similarly failed to replicate in mouse brain.
Fig. (1).
Influenza viruses replication in upper and lower respiratory tract and neuroivasions in mice at day 3 post infection (a) and day 6 post infection (b). Groups of four mice were intranasally inoculated with 6 log10 EID50 of each virus. Lung and nasal turbinates were titrated in chicken embryos starting from 1:10 initial dilution. Brain tissues were titrated starting from 1:2 initial dilution. Virus titers were expressed as log10 EID50/mL. Brain samples in which the virus wasn't isolated were estimated as limit of detection - 0.3 log10 EID50/mL. Data are represented as means infectious titers+ SD (error bars).
Vaccine Immunogenicity and Protection Studies in Mice
The H7N3 LAIV candidate was found to be immunogenic in mice (Table 1). One dose of 1000 MID50 or 100 MID50 H7N3 LAIV elicited HI antibody level with GMT of 8.7 and 6.6, respectively, the second dose of H7N3 LAIV at 21 dpi stimulated serum HI antibody levels to GMT 40.0 and 23.3 (for 1000 MID50 or 100 MID50 respectively).
Table 1.
Immunogenicity following immunization of mice with H7N3 LAIV
| Preparation | Mean HI titers (log2)±SD | |
|---|---|---|
| 21 dpi | 42 dpi | |
| H7N3 LAIV 1000 MID50 |
4.5±0.8 | 5.3±1.0 |
| H7N3 LAIV 100 MID50 |
2.7±0.5 | 3.1±0.4 |
| PBS | ≤2.3±0.0 | ≤2.3±0.0 |
Sera were tested for HI using homologous LAIV H7N3 virus as an antigen
Mean titers on day 42 were higher than those on day 21 (P = 0.043)
It was shown that all immunized animals survived after challenge with wild type A/mallard/Netherlands/12/2000 (H7N3) virus. Mice in all vaccine groups showed no signs of morbidity. It was demonstrated that the mice immunized with 1000 MID50 of H7N3 LAIV did not show significant loss in body weight for the period of 14 days after inoculation with wild virus (Table 2). At the end of the observation period an increase of 4% in animals body weight was observed compared to the initial weight. The group of mice immunized with 100 MID50 of the H7N3 LAIV showed weight loss by 5% on the 14th day after inoculation. The control non-immunized group of mice, starting from the 2nd day of observations, showed clinical signs of virus infection (hurried breathing, decrease in motion activity, aversion to food) followed by 9% body weight loss by the 6th day after challenge with the wild-type virus.
Table 2.
Protection in mice against challenge with 100 MID50 (~6 log10 EID50) of wild type virus A/Mallard/Netherlands/ 12/00(H7N3) following immunization with H7N3 LAIV1.
| Preparation | Survived on day 14 p.i./ total | Mean % of weight loss on day 6 p.i. | Virus isolation from tissues at day 3 pi | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lungs | Nasal turbinates | Brain | Spleen | |||||||
| No/ total | Virus titers (log10EID50/mL) | No/ total | Virus titers (log10EID50/mL) | No/ total | Virus titers (log10EID50/mL) | No/ total | Virus titers (log10EID50/mL) | |||
| H7N3 LAIV 1000 MID50 | 5/5 | 1 | 0/3 | ≤1.5±0.0 | 0/3 | ≤1.5±0.0 | 0/3 | ≤1.5±0.0 | 0/3 | ≤1.5±0.0 |
| H7N3 LAIV 100 MID50 | 5/5 | 6 | 0/3 | ≤1.5±0.0 | 0/3 | ≤1.5±0.0 | 0/3 | ≤1.5±0.0 | 0/3 | ≤1.5±0.0 |
| PBS | 5/5 | 9 | 3/3 | 5.7 ±1.3 | 3/3 | 4.2 ±0.3 | 0/3 | ≤1.5±0.0 | 0/3 | ≤1.5±0.0 |
Challenge was done 21 days after 2nd vaccine dose
P=0.049 compared to vaccinated mice
P=0.007 compared to vaccinated mice
As shown in Table 2, the challenge virus actively replicated in respiratory tissues of mock immunized animals (5.7 log10 EID50/mL in the lung and 4.2 log10 EID50/mL in the nasal turbinates), but failed to infect the brain and spleen. On the other hand, in both H7N3 LAIV vaccinated groups, all tested organs were free from presence of challenge virus. Thus, immunization of mice with either 1000 MID50 or 100 MID50 H7N3 LAIV protected the animals from the subsequent challenge infection with a homologous with wild type H7N3 virus.
Vaccine Safety in Chickens
During the 10 days period after intravenous introduction of H7N3 LAIV virus, none of the animals were registered with the signs of flu infection (Table 3). Thus the experimental batch of H7N3 LAIV was safe when administered intravenously to chickens.
Table 3.
Assessment of safety and infectious activity of H7N3 LAIV when administered intravenous or intranasal in chickens.
| Intravenous pathogenicity | Intranasal pathogenicity and infectivity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Morbidity (sick/ total) |
Mortality (dead/ total) |
Pathogenicity Index | Morbidity (sick/ total) |
Mortality (dead/ total) |
Virus recovery on the 3 dpi after intranasal inoculation with 6 log10 EID50/0.1 mL | Seroconversion on 14 dpi (ELISA) | |||||
| Swabs | Recovery from tissues | ||||||||||
| URP | Cloacae | Lungs | Heart | Kidneys | Brain | ||||||
| 0/8 | 0/8 | 0 | 0/8 | 0/8 | 0/10 | 0/10 | 0/2 | 0/2 | 0/2 | 0/2 | 0/8 |
Groups of eight chickens were infected intravenously with 8.1 log10 EID50/0.2 mL and observed daily for 10 days for clinical signs and death.
Groups of ten chickens were infected intranasally with 6 log10 EID50/0.1 mL. The oropharyngeal and cloacal swabs were collected 3 days p.i. and titrated in eggs for assessing viral replication. The chickens were observed for clinical signs of disease and death for 14 days. To determine infectivity, sera were collected 14 days p.i. and tested for the presence of antibodies by ELISA test
0 – absence of symptoms, 1 – development of infection signs, 2 – severe infection, 3 – fatal case
On the third day after intranasal application of 6,0 log10 EID50/0.1 mL dose of the vaccine, the H7N3 LAIV did not cause infection and vaccine virus was not recovered from upper respiratory tract (URP) or cloacal swabs. No virus recovered from the all analyzed tissues comprising lungs, kidneys, heart or brain. There was no seroconversion registered on the 14th day after intranasal application (Table 3). The effect of administration of the H7N3 LAIV to chickens was similar to previously reported effect of H2N2 MDV with low risk of infection [3].
Vaccine Safety and Attenuated Phenotype in Ferrets
Based on the data of virus recovery from respiratory organs of ferrets vaccinated with the H7N3 LAIV it was demonstrated that the vaccine, taken in two inoculating doses, similarly to control H1N1 LAIV, did not replicate in the lungs or nasal turbinates of the animals. At the same time, wild type virus H7N3 replication was evident as it was isolated from nasal turbinates of ferrets on the day 3 (Table 4).
Table 4.
Clinical symptoms and virus isolation from respiratory organs of ferrets after inoculation of H7N3 LAIV.
| Group No. | Preparation | Ferret No | Virus dose | Clinical symptoms on dpi after inoculation | Organs sampling, dpi | Virus isolation, PFU/0.1 g (log 10) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Lung | Nasal turbinates | |||||
| 1 | H7N3 LAIV | 1-3 | 106 | 0 | 0 | 0 | 0 | X | 3 | 0 | 0 |
| 4-6 | 106 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | ||
| 2 | H7N3 LAIV | 7-9 | 107 | 0 | 0 | 0 | 0 | X | 3 | 0 | 0 |
| 10-12 | 107 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | ||
| 3 | wt H7N3 | 13 | 107 | 0 | 0 | 0 | 0 | X | 3 | 0 | 1.7 |
| 14 | 107 | 0 | 0 | 0 | 0 | X | 5 | 1.9 | 1.9 | ||
| 15 | 107 | 0 | 0 | 0 | 0 | X | 3 | 0 | 3.7 | ||
| 16-18 | 107 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | ||
| 4 | LAIV H1N1 | 19-21 | 107 | 0 | 0 | 0 | 0 | X | 3 | 0 | 0 |
| 22-24 | 107 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | ||
0 – absence of clinical symptoms, X- no data
Studies of possible effects of H7N3 LAIV, administered in two inoculating doses, on infectious process and clinical symptoms in ferrets demonstrated its tolerance by the vaccinated animals and full absence of clinical symptoms of influenza in ferrets (Table 4). Furthermore, there were no clinical symptoms of the infection in the control groups of animals, which received H1N1 LAIV preparation or H7N3 wild type virus.
Changes in body weight and temperature in animals which received H7N3 LAIV did not differ animals of control groups, inoculated with H1N1 LAIV or wild type virus H7N3 (data not shown).
Based on the summarized data of body temperature and body weight time course, observed lack of clinical symptoms and virus isolation from respiratory organs of ferrets, it is possible to make a conclusion that monovalent H7N3 LAIV, prepared on the basis of attenuated MDV A/Leningrad/ 134/17/57 (H2N2), was well tolerated to ferrets and its replication profile is similar to attenuated H1N1 LAIV strain which has the same backbone.
Vaccine Immunogenicity and Protection Study in Ferrets
The animals immunized with H7N3 LAIV had the antibody levels (GMT 173-187) to homologous virus sufficient to protect them from subsequent infection with wild type virus (Table 5). The challenge wild type virus was recovered only in group of mock inoculated animals. The challenge virus was found in nasal washes of all animals in this group and in the lung of only one ferret on day 3 after challenge. On days 5, 7 and 9 challenge virus was not isolated from nasal washes of placebo group.
Table 5.
Humoral immune response in ferrets following H7N3 LAIV immunization and challenge virus recovery from respiratory tract after infection with 107 of wild type influenza H7N3 virus.
| Vaccine group | No.of ferrets | HI titers (GMT) after immunization : | Challenge on 14 days after second vaccine dose | Challenge virus isolation at day 3 pi | ||||
|---|---|---|---|---|---|---|---|---|
| Lungs | Nasal washes | |||||||
| One dose | Two doses | No/total | Mean virus titer (log10PFU ml-1 ±SD) | No/total | Mean virus titer (log10PFU ml-1 ±SD) |
|||
| H7N3 LAIV 107 EID50 | 6 | 23.3 | 186.7 | wt H7N3 107 EID50 | 0/3 | 0±0.0 | 0/3 | 0±0.0 |
| H7N3 LAIV 107 EID50 | 6 | 11.6 | 173.3 | PBS | 0/3 | 0±0.0 | 0/3 | 0±0.0 |
| PBS | 6 | ≤10.0 | ≤10.0 | wt H7N3 107 EID50 | 1/3 | 1.3±0.5 | 3/3 | 3.0±0.9 |
| PBS | 6 | ≤10.0 | ≤10.0 | PBS | 0/3 | 0±0.0 | 0/3 | 0±0.0 |
Groups of six ferrets received two administration of 107 EID50 H7N3 LAIV intranasally in the volume 0.5 ml 28 days apart (Day 0 and Day 28). HAI titers were measured for serum samples collected on day 28 and 42
GMTs on day 42 were higher than those on day 21 after immunization (P = 0.027)
Data of body weight and body temperature changes of vaccinated ferrets did not reveal significant differences in these parameters when compared with the control animals, administered placebo at first and second inoculation, except mock-vaccinated and challenge animals lost some weight, indicating decreasing appetite (data not shown).
No clinical symptoms were observed in ferrets, vaccinated with H7N3 LAIV. Furthermore, there were no clinical symptoms revealed when the vaccinated animals were inoculated with apathogenic wild type virus H7N3.
Histological Study in Ferrets
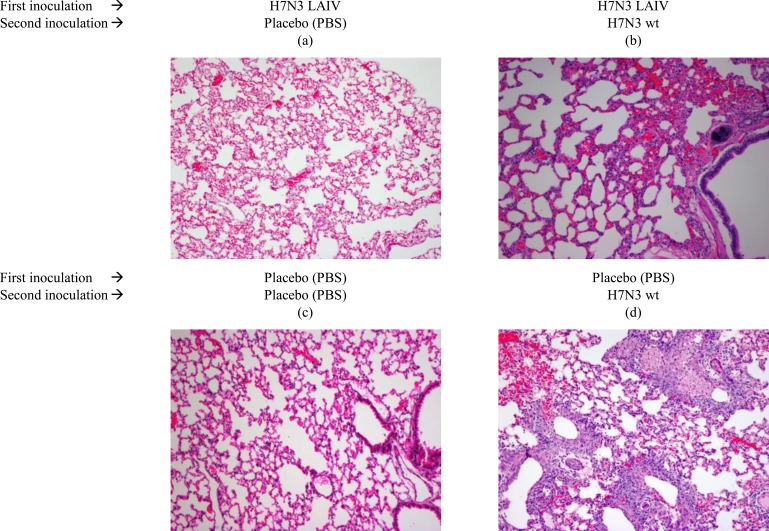
Fig (2). presents histological pattern of lungs tissues obtained from ferrets administered H7N3 LAIV or placebo after subsequent infection with wild type influenza H7N3 virus. Lung tissues, obtained from vaccinated ferrets (Fig. 2a, 2b), did not differ from that of the control animals (Fig. 2c), administered with placebo. Challenge with wild type H7N3 virus of LAIV-vaccinated animals, did not reveal any signs of abnormal changes in lung tissues (Fig. 2b). Fig. (2d) presents inflammation in the lung of mock-vaccinated animal inoculated with wild type influenza H7N3 virus on day 3 pi. Thus, the H7N3 LAIV provided protection from lung infection with wild type influenza H7N3 virus in ferrets.
Fig. (2).
Histological pattern of lungs tissues of ferrets administered H7N3 LAIV or placebo after subsequent infection with wild type influenza H7N3 virus. Groups of six ferrets received two administration of 107 EID50 H7N3 LAIV intranasally in the volume 0.5 mL 28 days apart (Day 0 and D28). On day 42 the animals were challenged intranasally with 106 EID50 of wild type influenza virus A/mallard/Netherlands/12/2000 (H7N3) in volume of 0.1 mL. Control animals received 0.1 mL of physiological saline intranasally. Three animals from each group were euthanized on the day three after experimental challenge, and three remaining ferrets euthanized on the day 14. One lung from each sacrificed animal was used for histological analysis. Sections were stained with haematoxylin and eosin (200x magnification).
DISCUSSION
The mouse and ferret models widely used to better understand the pathogenicity of avian influenza viruses for mammalian species, are generally able to predict the pandemic potential of such viruses, and are helpful in the development of improved methods for the prevention and control of the virus in a potential pandemic [11]. Moreover, in case of H7 viruses challenge study of efficacy of influenza vaccines cannot be ethically conducted in humans.
A subset of the H7 viruses was evaluated for their ability to replicate and cause disease in BALB/c mice following intranasal administration. H7 subtype viruses are able to infect mice without adaptation and manifested different levels of lethality and kinetics of replication [12]. The A/Netherland/219/03 H7N7 virus, as well as some avian H5N1 viruses, spread systemically following ocular inoculation, including to the brain, resulting in morbidity and mortality of mice. This data correlated with efficient replication of highly pathogenic H7 and H5 subtypes in murine corneal epithelial sheets (ex vivo) and primary human corneal epithelial cells (in vitro) [13].
A number of H7-based vaccines have been developed and evaluated in preclinical studies on different animal models [11-18]. Subunit influenza vaccine based on recombinant influenza virus rPR8-H7N7 developed by reverse genetics and containing HA from A/Mallard/ Netherlands/12/00 (H7N3) and NA from A/Netherlands/ 33/03 (H7N7) was tested in the mouse model. The efficacy of classical non-adjuvanted subunit vaccine and an immune stimulatory complex-adjuvanted vaccine was compared. Mice receiving the non-adjuvanted vaccine revealed low antibody titers, lack of clinical protection, high virus titers in the lungs, and presence of challenge virus in the spleen, liver, kidney and brain. In contrast, mice receiving two doses of the adjuvanted vaccine revealed high antibody titers, clinical protection from the infection, ~ 1,000-fold reduction of virus titers in the lungs, and occasional detection of challenge virus in other organs. This was the first report of an H7 vaccine candidate tested in mammalian model [14].
Another H7N7/PR8 vaccine reassortant virus was generated containing surface glycoprotein genes from mallard influenza viruses and internal genes of A/Puerto Rico/8/34 (H1N1) strain. Antigenic analysis revealed that the vaccine candidate virus confered broad antigenic cross-reactivity against contemporary Eurasian and the North American H7 subtype human isolates. Mice immunized with formalin inactivated (FI) H7N7/PR8 whole virus vaccine with or without aluminum hydroxide adjuvant were clinically protected from mortality and had reduced pulmonary replication of the A/Netherlands/219/03 (H7N7) challenge virus. The FI H7N7/PR8 whole virus vaccine also afforded cross-protection in mice at the pulmonary level against antigenically distinct North American A/Canada/ 444/04 (H7N3) human isolate. This vaccine candidate virus satisfied the agricultural safety requirements for chickens, proved safe in mice and has entered in phase I human clinical trials [15].
A live attenuated H7N7 candidate vaccine virus was generated by reverse genetics using the modified hemagglutinin (HA) and neuraminidase (NA) genes of highly pathogenic (HP) A/Netherlands/219/03 (NL/03) (H7N7) wild type (wt) virus and the six internal protein genes of the cold-adapted (ca) A/Ann Arbor/6/60 (H2N2) master donor virus. The reassortant H7N7 NL/03 ca vaccine virus was temperature sensitive and attenuated in mice, ferrets and African green monkeys. Intranasal (i.n.) administration of a single dose of the H7N7 NL/03 ca vaccine virus fully protected mice from lethal challenge with homologous and heterologous H7 viruses from Eurasian and North American lineages. Two doses of the H7N7 NL/03 ca vaccine induced neutralizing antibodies in serum and provided complete protection from pulmonary replication of homologous and heterologous wild type H7 challenge viruses in mice and ferrets. One dose of the H7N7 NL/03 ca vaccine elicited an antibody response in one of three African green monkeys that was completely protected from pulmonary replication of the homologous wild type H7 challenge virus. The contribution of CD8+ and /or CD4 T cells to the vaccine-induced protection of mice was evaluated by T-cell depletion; T lymphocytes were not essential for the vaccine-induced protection from lethal challenge with H7 wt viruses. Passively transferred neutralizing antibody induced by the H7N7 NL/03 ca virus protected mice from lethality following the challenge with H7 wt viruses [16].
In our current study we have demonstrated that H7N3 LAIV candidate generated from LP avian strain A/mallard/Netherlands/12/2000 (H7N3) is indistinguishable from the parental MDV with regards to replication kinetic in the upper and lower respiratory tract of mice. The safety, immunogenicity and protection of H7N3 LAIV were tested in different animal models. Following administration of H7N3 LAIV to mice and ferrets recoverable virus was detected only from upper respiratory tract. The immune response was achieved after two vaccine doses.
Since February 2013 the emergence of A(H7N9) influenza in humans caused a major public health concern about pre-existing immunity to this subtype in the population including those after immunization with current influenza vaccine. The ferret immunogenicity study conducted with the H7 LAIVs based on A/Ann Arbor/6/60 master strain had shown that the H7N3 and H7N7 vaccine immunized ferret sera cross reacted in HI test with the newly emerged H7N9 virus. Based on the cross protection data obtained from the H7N7 against H7N3 wt virus challenge, the authors predicted that the existing H7N3 or H7N7 ca vaccines would provide some degree of protection against H7N9 virus [18]. Our previous study with LAIV against potentially pandemic influenza viruses demonstrated that mice infected intranasally with one dose of H5N2 LAIV a high level of cross-protection from severe disease, death and challenge virus H5N1 VN/1203 replication even though the vaccine and challenge strains shared an HA1 subunit amino acid identity of only 91%. This cross-protective effect was observed in mice with low or undetectable levels of neutralizing antibodies against the challenge virus H5N1 VN/1203 [19]. Comparison with the newly isolated H7 influenza viruses had shown a 96-97 % identity of the HA1 subunit amino acid sequences between the reported in present study H7N3 vaccine strain and the 2013 H7N9 influenza viruses (data not shown).
We demonstrated that in ferrets H7N3 LAIV could not be isolated from respiratory organs. Our study also had shown that the H7N3 LAIV candidate was immunogenic and fully protected mice and ferrets against subsequent challenge with the homologous wild type virus. The study in chickens showed complete safety and lack of replication of this preparation.
Thus, in our study we confirmed that H7N3 LAIV candidate was safe in mice, ferrets and chickens, raised serum post immunization antibody response and provided protection from the influenza infection in appropriate animal models. Based on the preclinical data an approval for a phase I clinical study was obtained from Russian Public Health authorities. The results of that study will be reported separately.
ACKNOWLEDGMENTS
This study was performed and supported as part of the Development and Collaboration Agreement between Institute of Experimental Medicine and PATH Vaccine Solutions. We are grateful to Dr. Rick Bright and Dr. Kathy Neuzil for their support in this study and discussions. The authors would like to thank Vadim Tsvetnitsky for useful discussions and editing assistance.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Ambrose CS, Luke C, Coeling K. Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza. Influenza Other Respir Viruses. 2008;2(6):193–202. doi: 10.1111/j.1750-2659.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fouchier RA, Schneeberger PM, Rozendaal FW , et al. Avian influenza A virus (H7N7):associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci USA. 2004;101 (5):1356–61. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz JM, Desheva JA, Rudenko LG, Rekstin AR, Swayne DE, Cox NJ, Klimov AI. Development of candidate H7N3 live attenuated influenza vaccine Proceedings of the Options for the control of influenza VI. Toronto June 17-23. 2007 Int. Med. Press Lndon Atlanta. 2008:591–2. [Google Scholar]
- 4.Wareing MD, Marsh GA, Tannock GA. Preparation and characterisation of attenuated cold-adapted influenza A reassortants derived from the A/Leningrad/134/17/57 donor strain. Vaccine. 2002;20(16):2082–90. doi: 10.1016/s0264-410x(02)00056-7. [DOI] [PubMed] [Google Scholar]
- 5.Klimov AI, Cox NJ. PCR restriction analysis of genome composition and stability of cold-adapted reassortant live influenza vaccines. J Virol Methods. 1995;52(1-2):41–9. doi: 10.1016/0166-0934(94)00133-2. [DOI] [PubMed] [Google Scholar]
- 6.Rudenko L, van den Bosch H, Kiseleva I , et al. Live attenuated pandemic influenza vaccine clinical studies on A/17/California/2009,/38 (H1N1):and licensing of the Russian-developed technology to WHO for pandemic influenza preparedness in developing countries. Vaccine. 2011;29 (Suppl 1 ):A40–4. doi: 10.1016/j.vaccine.2011.04.122. [DOI] [PubMed] [Google Scholar]
- 7.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 8.Lu X, Tumpey TM, Morken T, Zaki SR, Cox NJ, Katz JM. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1):viruses isolated from humans. J Virol. 1999;73:5903–11. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subbarao K, Chen H, Swayne D , et al. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology. 2003;305 (1):192–200. doi: 10.1006/viro.2002.1742. [DOI] [PubMed] [Google Scholar]
- 10.Webster RG, Cox NJ, Stohr K. WHO manual on animal influenza diagnosis and surveillance World Health Organization, Geneva, Switzerland. http: //www.who.int/csr/resources/ publications/influenza/whocdscsrncs20025rev.pdf. 2002.
- 11.Belser JA, Szretter KJ, Katz JM, Tumpey TM. Use of animal models to understand the pandemic potential of highly pathogenic avian influenza viruses. Adv Virus Res. 2009;73:55–97. doi: 10.1016/S0065-3527(09)73002-7. [DOI] [PubMed] [Google Scholar]
- 12.Joseph ?, McAuliffe J, Lu B, Jin H, Kemble G, Subbarao K. Evaluation of replication and pathogenicity of avian influenza A H7 subtype viruses in a mouse model. J Virol. 2007;81(19):10558–66. doi: 10.1128/JVI.00970-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belser JA, Wadford DA, Xu J, Katz JM, Tumpey TM. Ocular infection of mice with influenza A (H7):viruses a site of primary replication and spread to the respiratory tract. J Virol. 2009;83(14):7075–84. doi: 10.1128/JVI.00535-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Wit E, Munster VJ, Spronken MIJ , et al. Protection mice against lethal infection with highly pathogenic H7N7 influenza A virus by using a recombinant low-pathogenicity vaccine strain. J Virol. 2005;79(19):12401–7. doi: 10.1128/JVI.79.19.12401-12407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadhao SJ, Achenbach J, Swayne DE, Donis R, Cox N, Matsuoka Y. Development of Eurasian H7N7/PR8 high growth reassortant virus for clinical evaluation as an inactivated pandemic influenza vaccine. Vaccine. 2008;26(14):1742–50. doi: 10.1016/j.vaccine.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 16.Pappas C, Matsuoka Y, Swayne DE, Donis RO. Development and evaluation of an influenza virus subtype H7N2 vaccine candidate for pandemic preparedness. Clin Vaccine Imunol. 2007;14(11):1425–32. doi: 10.1128/CVI.00174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min JY, Vogel L, Matsuoka Y , et al. A live attenuated H7N7 candidate vaccine virus induces neutralizing antibody that confers protection from challenge in mice, ferrets and monkeys. J Virol. 2010;84(22):11950–60. doi: 10.1128/JVI.01305-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Q, Chen Z, Cheng X, Xu L, Jin H. Evaluation of live attenuated H7N3 and H7N7 vaccine viruses for their receptor binding preferences, immunogenicity in ferrets and cross reactivity to the novel H7N9 virus. PLoS One. 2013;8(10):e76884. doi: 10.1371/journal.pone.0076884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu X, Edwards LE, Desheva JA , et al. Cross-protective immunity in mice induced by live-attenuated or inactivated vaccines against highly pathogenic influenza A (H5N1):viruses. Vaccine. 2006;24(44-46):6588–93. doi: 10.1016/j.vaccine.2006.05.039. [DOI] [PubMed] [Google Scholar]