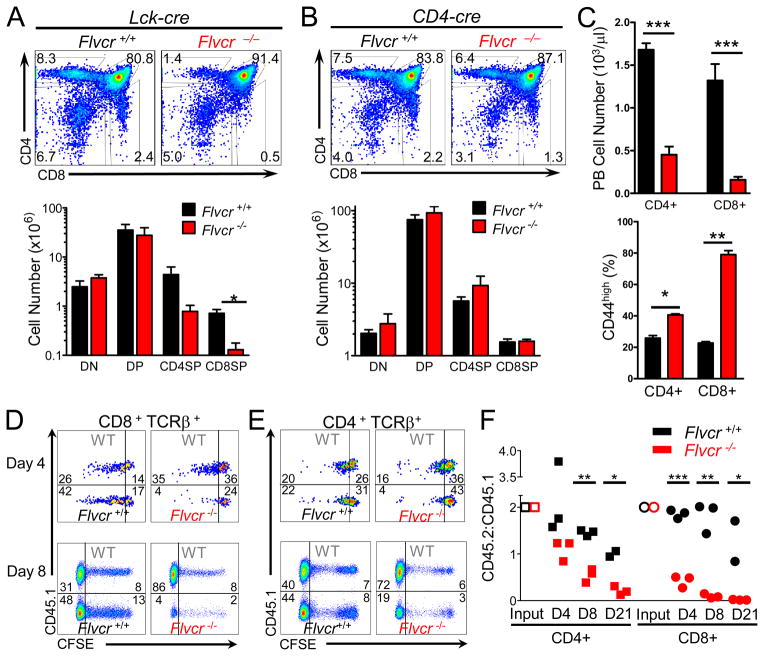
FIGURE 4.
FLVCR is required for peripheral T cell survival. (A) CD4 and CD8 staining of thymocytes from Flvcrflox/flox;Lck-cre and control mice (upper) with absolute cell numbers of thymus subsets shown in lower panel. *p=0.007. (B) CD4 and CD8 staining of thymocytes of Flvcrflox/flox;CD4-cre and control mice (upper) with absolute cell numbers of thymus subsets shown below. (C) Absolute numbers of CD4+ and CD8+ peripheral blood T lymphocytes in Flvcrflox/flox;CD4-cre and control mice (upper). Percentages of CD4+ and CD8+ peripheral blood T lymphocytes from Flvcrflox/flox;CD4-cre and control mice with high CD44 surface expression (lower). *p=0.003 **p=0.0005 ***p≤0.0001. (D–E) CFSE-labeled Flvcrflox/flox;CD4-cre or Flvcr+/+;CD4-cre thymocytes were mixed at a ratio of 2:1 with CD45.1 (WT) control thymocytes and adoptively transferred into sublethally irradiated Rag1−/− mice. 4 and 8 days later, spleens were harvested and CFSE dilution of transferred thymocytes was analyzed by flow cytometry. Dot plots are gated on CD8 + TCRβ + (D) or CD4 + TCRβ+ cells (E). Inset numbers show frequency of cells in each gate. (F) CD45.2:CD45.1 ratio of adoptively-transferred SP thymocytes and splenic lymphocytes at days 4 and 8 post-transfer. CD45.2:CD45.1 ratios were normalized to the CD45.2:CD45.1 ratio of the input CD4SP or CD8SP. *p<0.03 **p<0.0008 ***p<0.0001. Data are representative of 4 separate experiments with 4–6 mice per group (A–C). Data are representative of 3 separate experiments with 10–12 mice per group total (D–F).