Abstract
Buffy coats are the most common method for the acquisition of activated primary human T cells for research or clinical applications, but recently leukocyte reduction system (LRS) cones have emerged as a viable source for these cells. In this study, we determined if activated human T cells derived from buffy coats or LRS cones had different functionality. No changes in the expression of surface receptors were observed except for a significant increase in CD44 expression on T cells isolated from LRS cones. LRS cone-derived T cells trended towards higher receptor-mediated cytokine production and had significantly increased donor-to-donor variability in IFN-γ production. TCR-induced ERK1/ERK2 and AKT phosphorylation was also increased in T cells isolated from LRS cones. In conclusion, LRS cones are an excellent source of T cells for clinical and research applications, but these cells have subtle functional differences from T cells isolated using standard buffy coats.
Keywords: T cells, T cell receptor, Leukocyte reduction systems, buffy coat
1. Introduction
Human T cells are required for the adaptive immune response to infection but their inappropriate function can drive the pathogenesis of numerous diseases. Research on the activation and function of human T cells is critical not only for a better understanding of the adaptive immune response and the development of pathological diseases, but also for the development of effective vaccines and treatments for T cell-mediated disease. Additionally, human T cells are needed for several evolving clinical applications, including the expansion of tumor infiltrating lymphocytes or production of T cells with chimeric antigen receptors [1, 2]. Many experimental investigations and clinical protocols require substantial quantities of peripheral blood mononuclear cells (PBMCs) to generate T cells, numbers that can be difficult to acquire via the widely used buffy coat isolation. Several recent studies have shown that large numbers of PBMCs, often >1 × 109 cells, can be isolated from leukocyte reducing system (LRS) cones [3–6], which are used to reduce transfusion reactions, reactions to alloantigens in transplant patients and transmission of pathogens that infect leukocytes. Therefore, LRS cones are emerging as an efficient, safe and convenient asset to many research and clinical applications that require large numbers of human T cells.
Several groups have examined the phenotype and function of immune cells isolated from LRS cones compared to standard buffy coats. They observed that the percentage of T cells, B cells, monocytes and dendritic cells was largely similar between PBMCs isolated from LRS cones or buffy coats, but that PBMCs from LRS cones contain more granulocytes [3–5, 7]. Dendritic cells isolated from LRS cones were functional and capable of activating T cell responses [3, 6]. B cells isolated from LRS cones have similar expression of surface markers and ability to be activated through CD40 compared to B cells isolated from buffy coats [5]. Less is known about the functionality of T cells isolated from LRS cones compared to buffy coats. T cells isolated using leukocyte filters, which are structurally different from LRS cones, have reduced Staphylococcal enterotoxin B (SEB)-induced CD69 or CD25 expression on CD3+ cells compared to buffy coat T cells[4]. However, T cells isolated using LRS cones have enhanced SEB-mediated upregulation of CD69 or CD25 compared to T cells isolated from leukocyte filters, but appear to have similar SEB responses to buffy coat isolated cells [7] Together, these studies suggest that the T cells may be functionally different depending on the source of the PBMCs from which these cells are isolated. However, it is currently unknown whether the downstream function, such as cytokine production and early signaling events, of T cells isolated from LRS cones is comparable to the functions of T cells isolated from standard buffy coats. Additionally, no studies have examined whether the source of PBMCs alters the subsequent function of T cells that are expanded from these cells. Thus we further investigated the functionality of T cells derived from PBMCs isolated using LRS cones by examining cell surface receptor expression, cytokine release, and phosphorylation of early signaling pathways compared to buffy coat isolation. We observed that T cells isolated from LRS cones had similar expression of surface markers except for CD44, increased production and donor-to-donor variability in IFN-γ production, and enhanced TCR-mediated ERK1/ERK2 and AKT activation. Together, these data suggest that LRS-derived T cells have subtle functional differences compared to T cells isolated from buffy coat.
2. Materials and Methods
2.1. Reagents
Anti-ERK1/ERK2 pT185/pY187, anti-AKT pS473 antibodies and dynabeads were purchased from Life Technologies, Grand Island, NY, USA. The polyvinylidene difloride (PVDF) membrane and anti-actin antibodies were obtained from EMD Millipore, Billerica, MA, USA. RPMI 1640, L-glutamine, penicillin-streptomycin and PBS were acquired from Gibco, Grand Island, NY, USA. The FBS was purchased from Atlanta Biologicals, Flowery Branch, GA, USA. The anti-CD3 antibody (OKT3), anti-CD4 antibody (RPA-T4), PE anti-human CD8a antibody (RPA-T8), PE/Cy5 anti-human CD69 antibody (FN50), PE anti-human TCR α/β T cell receptor (IP26), FITC anti-human CD28, anti-human CD28 antibody (CD28.2), recombinant human IFN-γ, purified anti-human IFN-γ and biotin anti-human IFN-γ were purchased from Biolegend, San Diego, CA, USA. The PE/Cy5 anti-human CD62L antibody (DREG-56) and the PE/Cy5 anti-human CD45RO antibody (UCHL1) were obtained from BD Pharmingen, San Jose, CA, USA. Streptavidin-HRP was purchased from Jackson Immuno Research Labs, West Grove, PA, USA. The anti-human CD44 antibody (IM7), purified anti-human IL-2 and biotin anti-human IL-2 were acquired from eBioscience, San Diego, CA, USA. Recombinant IL-2 was obtained from R & D Systems, Minneapolis, MN, USA. Human rIL-2 was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: Human rIL-2 from Dr. Maurice Gately, Hoffman—La Roche Inc. ELISA tetramethylbenzidine peroxidase substrate was purchased from Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA. The Criterion polyacrylamide gels were acquired from Bio-Rad, Hercules, CA, USA. SEA BLOCK blocking buffer was purchased from Thermo Scientific, Waltham, MA, USA. All chemicals were research grade and obtained from a variety of sources.
2.2. Standard Buffy Coat Isolation
Human PBMCs were isolated from heparin anti-coagulated venous blood from healthy, consenting adults. The PBMCs isolated from the standard buffy coat protocol were from participants who had given informed consent for an Institutional Review Board (IRB) approved study at the University of Iowa. In these studies, the PBMCs were not needed to complete the IRB approved studies and were normally discarded. Because all cells used in these studies were obtained from normally discarded products, the donors had approved for the use of their cells in research projects and the donors were completely de-identified, these studies were exempt from further IRB approval. The donors were generally recruited from the students and staff at the University of Iowa and ranged in age from 21–40 years old. PBMCs were isolated using Hypaque-Ficoll density-gradient separation, followed by removal of the buffy coat. The PBMCs were then washed three times with 1X PBS, before dilution in complete RPMI media (RPMI 1640 supplemented with 10% FBS, 2mM L-glutamine, and 50 mg/mL streptomycin-50 U/mL penicillin).
2.3. Leukocyte Reducing System (LRS) Cone Isolation
Human PBMCs were obtained from whole blood from anonymous donors from the DeGowin Blood Center at the University of Iowa. The age of the blood donors were unknown but restricted to donors under the age of 55. The blood donors had consented to allow blood cells not used for donation to be used for research at the University of Iowa. The consent process and consent documents for these donors have been approved by the IRB for the University of Iowa. LRS cones from a Trima Accel automated blood collection system (Terumo BCT, Lakewood, CO, USA) were used to remove PBMCs from these blood products, and the LRS cones were provided to investigators at the University of Iowa by the DeGowin blood center. The PBMCs from the LRS cones were flushed from the cone by washing with isolation buffer (PBS containing 2 mM EDTA and 2% FBS) and further isolated using Hypaque-Ficoll density-gradient separation. The PBMCs were then washed three times with 1X PBS, before dilution in complete RPMI media.
2.4. Growth of PBMCs
The PBMCs from standard buffy coat isolation and LRS cone isolation were incubated at 37 degrees in complete RPMI media for 2–4 hours to remove adherent cells. The non-adherent cells were then cultured at 2–4 × 106 cells/ml in complete RPMI with 100 U/mL recombinant IL-2. To enrich for T-cells, the cells were activated with magnetic Dynabeads coated with anti-CD3 and anti-CD28 for 5 days before using in experiments.
2.5. Flow Cytometry
After five days of activation, the anti-CD3/anti-CD28 coated magnetic beads were removed from the cells. The cells were resuspended to a concentration of 4 × 105 cells/mL in FACS buffer (PBS with 10% FBS and 0.05% sodium azide). For single stains, an isotype control or a single indicated primary antibody was added and cells incubated on ice for 30 minutes. The cells were then washed extensively with FACS buffer and, if needed, a secondary antibody was added for 30 minutes on ice. For double staining, the two antibodies, each directly conjugated to separate fluorophores, were incubated with cells on ice for 30 minutes, then washed extensively with FACS buffer. Samples were collected using an Accuri C6 flow cytometer and the data was gated and analyzed using software provided by Accuri (BD Biosciences, San Jose, CA, USA). Live lymphocytes were gated based on forward and side scatter and the gates were made such that the percent positive cells in the isotype control was between 4 and 5%. The same gates were then used for isolated cells stained with the individual antibodies. The gates for the double stained cells were based on gates set for the single stained cells in the same experiment. The percent positive cells by a specific antibody in the gated area of live cells and the median fluorescence intensity of all cells were graphed using Prism (Graphpad Software, La Jolla, CA, USA).
2.6. TCR-induced Cytokine Production
Activated peripheral blood T cells (1 × 106/ml) were stimulated for 48 hours at 37 C in supplemented RPMI with or without 2 μg/ml plate-bound anti-CD3 and in absence or presence of 1 μg/ml anti-CD28, 1 μg/ml Fibronectin or 1 μg/ml anti-CD44. Supernatants were collected and stored at −20°C. IL-2 and IFN-γ levels in an individual culture supernatant were measured in triplicate by standard TMB based ELISA using an Epoch plate reader at 450 nM (BioTek, Winooski, VT, USA). Six to eight independent donors were characterized for each experiment and the data was then plotted using the graphing program Prism (Graphpad Software, La Jolla, CA, USA).
2.7. Stimulation of Peripheral Blood T cells
After five days of activation, the activated peripheral blood T cells were washed with RPMI 1640 and resuspended to a concentration of 2 × 107 cells/ml in RPMI 1640. The cells were treated with anti-CD3 (10 μg/ml) and anti-CD4 (2 μg/ml) for 30 minutes on ice. Since concurrent stimulation with CD3 and CD4, not CD3 stimulation alone, could induce detectable TCR-mediated signaling [8] both antibodies were used. The cells were warmed to 37°C for 10 minutes and stimulated with anti-mouse IgG (25 μg/ml) for various times. The cells were lysed with a 4-fold excess of hot 2X lysis buffer, heated to 95°C for 4 minutes and sonicated to reduce viscosity.
2.8. Immunoblotting and Analysis
The cellular lysates were separated by polyacrylamide gel electrophoresis (PAGE) using 4–15% Criterion gels and the proteins were transferred to PVDF. The membranes were blocked for 1 hour at room temperature in a blocking buffer consisting of 50% PBS and 50% SEA BLOCK Blocking Buffer. Primary antibodies were added to blocking buffer at their appropriate dilutions and then incubated with the membrane overnight at 4°C. After washing twice with PBST (PBS + 0.1% Tween 20), the secondary antibodies were added in blocking buffer for 30 minutes at room temperature. The membranes were washed room temperature with PBST with 10% SDS and then 2 times with PBST alone. Antibody binding was detected using a Licor Odyssey (Lincoln, NE, USA). Six independent replicates were performed for each experiment. The intensity of the immunoblotting bands was determined using the Licor Oddessey v3.0 software. The amount of phosphorylation, as determined by the intensity of phosphospecific antibody immunoblots, were normalized to the relative amount of the analyzed protein in the samples, as determined by the ratio of the intensity of the pan ERK1/ERK2 or AKT antibody and actin antibody immunoblots. Fold activation for each sample was calculated by dividing the normalized intensity for the individual point with the normalized intensity for the 0 minute timepoint. The average normalized intensities and fold activation from six independent experiments using different donors were calculated and plotted using the graphing program Prism (Graphpad Software, La Jolla, CA, USA).
2.9. Statistical Analysis
Due to the inherent variability of human subjects and to eliminate the possible bias of obvious outliers, the outliers in the data were identified using the ROUT method with Q set at 1%, which is the default values used by [9]. Statistical differences between various samples in the flow cytometry and immunoblotting experiments were determined using two-tailed unpaired t tests. The cytokine samples were statistically compared using two-tailed unpaired t tests with Welch’s correction due to differences in the variability of the samples [10]. Statistical differences between variance in these samples were determined by F test to compare variance with statistical significance set at a more stringent p< 0.01 due to the inherent unreliability of this statistical test. All statistical analysis was repeated using the non-parametric Mann-Whitney test. As noted in the text, the only difference between the two tests was the p values for the percent of cells positive for CD44, where p=0.048 for the t test and p=0.055 for the Mann-Whitney test, and CD44 MFI between CD45RO+ versus CD45RO− T cells, where p=0.030 for the t test and p=0.057 for the Mann-Whitney test. All values shown on the figures are for the comparisons using the standard t tests.
3. Results
3.1. Expression of select cell surface receptors are different in activated peripheral blood T cells isolated from LRS cones
Previous studies have shown that similar percentages of CD4+ and CD8+T cells are present in PBMCs isolated from LRS cones or standard buffy coats [4, 5, 7]. However, no studies have examined whether expansion of T cells from PBMCs isolated from either LRS cones or buffy coats have differences in the percentages of cells expressing T cell markers. This is a critical question since the use of anti-CD3/anti-CD28 antibodies and recombinant IL-2 is a standard protocol to expand human T cell populations for clinical or research use. To this end, PBMCs were isolated from both standard buffy coats and LRS cones. PBMCs obtained from both sources were isolated using Hypaque-Ficoll density-gradient separation; thus, the only difference in isolation was filtration through the LRS cone. Once isolated, the PBMCs from both sources were activated using anti-CD3/anti-CD28 and IL-2 for five days. There were no differences in the relative amount of T cell expansion or T cell death/apoptosis between the two sources of PBMCs (data not shown). This suggests that the source of PBMCs does not greatly alter the subsequent proliferation of T cells.
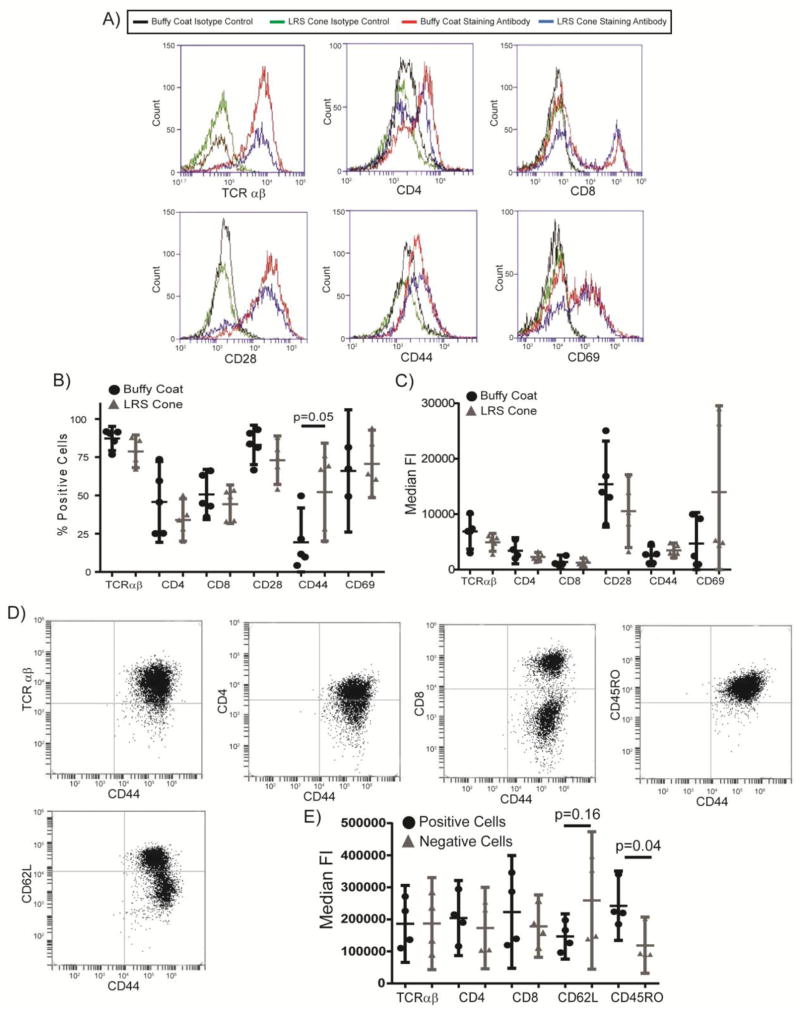
After expansion, the expression of surface markers was examined by flow cytometry. Both PBMC populations contained greater than 75% T cells (Figure 1). Staining with CD19 suggested that the vast majority of the contaminating cells were B cells, with little to no contamination from monocytic cells (data not shown). There were no significant differences in the percentages of cells expressing the TCRα/β chain, CD4, CD8, CD28 and CD69 in activated PBMCs from LRS cones or buffy coats (Figure 1A). There a moderately significant increase in the percentage of cells expressing CD44 in activated PBMCs from LRS cones compared to buffy coat T cells (Figure 1A and 1B), with the t test having a p=0.048 and the Mann-Whitney non-parametric test having a p=0.055. None of the populations showed significant differences in the variability of the samples as determined by f tests to compare variances (data not shown).
Figure 1.
Activated T cells from standard buffy coat isolation and LRS cone isolation have similar surface receptor expression. (A) The expression of surface markers on expanded T cells from the two isolation methods was assessed by flow cytometry. (B) The percentage of cells expressing surface markers was determined by flow cytometry after setting the isotype control stained cells to 4–5% positive. The bar is the average ± the 95% confidence interval for six separate donors for each isolation methods with the outliers removed as described in the Materials and Methods. The p value for significant changes in expression of CD44 is shown. (C) The median fluorescence intensity of live cells was determined by flow cytometry. The results are an average ± 95% confidence intervals for six donors for each isolation method with the outliers removed as described in the Materials and Methods. No significant changes were observed. (D) The co-expression of surface markers on expanded T cells from the LRS cones was assessed by flow cytometry. (E) The median fluorescence intensity of CD44 in live cells that were positive or negative for the indicated surface protein was determined by flow cytometry. The results are an average ± 95% confidence intervals for four donors. The p value for differences in expression of CD44 is shown.
To further examine relative protein expression per cell of the various receptors, the median florescence intensity (MFI) of the live cells was determined. T cells produced from either LRS cones or standard buffy coats had similar MFI for TCRα/β chain, CD4, CD8, CD28, CD44, and CD69 (Figure 1A and 1C). There were also no statistically significant differences in the variation for any of the receptors. Additionally, we further characterized the phenotype of the CD44 positive cells produced from LRS cones. Greater than 90% of the cells from the LRS cones were positive for TCR αβ (Figure 1A and 1D). There was no difference in the MFI for CD44 in TCR αβ+ vs TCR αβ− cells (Figure 1D and 1E), showing conclusively that the increase in percentage of CD44+ cells in LRS cones was not due to contamination from non-T cells. Similarly, there was no difference in the MFI for CD44 in CD4+ versus CD4− or CD8+ versus CD8− T cells (Figure 1D and 1E). Interestingly, there was a trend for increased CD44 MFI in CD62L− T cells (p=0.16) and a significant increase in CD44 MFI in CD45 RO+ T cells (t test p=0.030 and Mann-Whitney non-parametric test p=0.057) (Figure 1D and 1E). This shows that the increased CD44 expression is occurring in activated T cells. Collectively, these data suggest that surface cell receptor expression is not substantially altered on T cells isolated using standard buffy coat or LRS cone isolation methods. The only observed differences were a significantly higher expression of CD44 on CD62L−/CD45RO+ activated T cells isolated from LRS cones.
3.2. IFN-γ production is altered in T cells isolated from LRS cones
TCR activation leads to increased production and release of cytokines important for the host immune response. These responses are enhanced by the concomitant activation of costimulatory and adhesion receptors, such as CD28, CD44 and the α4β1 integrin VLA-4. No studies have characterized potential differences in cytokine production from the different T cell isolation methods in expanded T cell populations. To address this knowledge gap, the activated T cells from standard buffy coat or LRS cone isolations were stimulated with plate-bound anti-TCR alone or in combination with soluble stimulatory anti-CD28, plate-bound fibronectin (a ligand for α4β1 integrin VLA-4), or plate-bound anti-CD44. Cells isolated from either buffy coat or LRS cones had increased IL-2 production when co-stimulated via CD28, VLA-4 or CD44 compared to stimulation through the TCR alone (Figure 2A). There were no significant differences in the production of IL-2 between the two isolation methods, although there was a trend (p=0.08) towards an increased production of IL-2 upon stimulation with TCR and fibronectin together in T cells isolated from LRS cones (Figure 2A). In contrast to IL-2 production, TCR stimulation alone increased IFN-γ production in the LRS cone or buffy coat isolated T cells. Costimulation with CD28, fibronectin, and CD44 resulted in only a moderate enhancement of IFN-γ production in both isolation methods (Figure 2B). There was a trend towards significant increase in IFN-γ production in TCR alone (p= 0.13), TCR/CD28 (p= 0.09), TCR/VLA-4 (p= 0.10), and TCR/CD44 (p= 0.14) in T cells isolated from LRS cones compared to buffy coats (Figure 2A). Interestingly, as assessed by the f test to compare variances, there was significantly enhanced variation between donors in LRS cone derived T cells treated with TCR/CD28 (p= 0.001), TCR/VLA-4 (p= 0.0012), and TCR/CD44 (p= 0.0059) compared to buffy coat isolated T cells when stimulated via these same receptors (Figure 2B). These data suggest that isolation of T cells by LRS cones results in enhanced levels and donor-to-donor variation of receptor-mediated production of IFN-γ compared to T cell isolated from a standard buffy coat.
Figure 2.
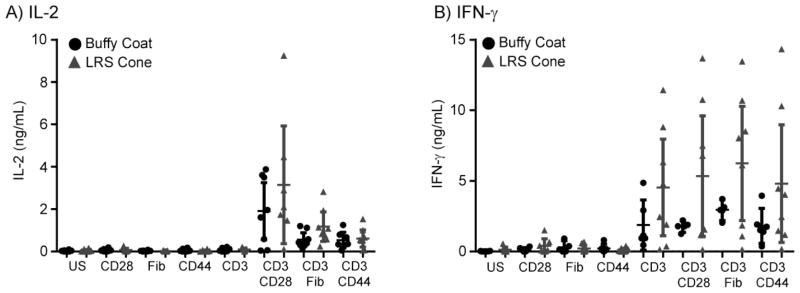
Cytokine production and donor-to-donor variability is increased in the LRS cone isolated cells. T cells isolated from buffy coats or LRS cones were unstimulated (US) or stimulated with plate-bound anti-human CD3 (2 μg/ml) in the absence or presence of anti-CD28 (1 μg/ml), Fibronectin (1 μg/ml) or anti-CD44 (1 μg/ml) for 48 hours at 37 degrees. Supernatants were collected and assayed by ELISA for (A) IL-2 production and (B) IFN-γ production. The averages ± SEM of six different donors for each isolation method are shown with the outliers removed as described in the Materials and Methods.
3.3. Early TCR-mediated signaling is enhanced in T cells derived from LRS cone
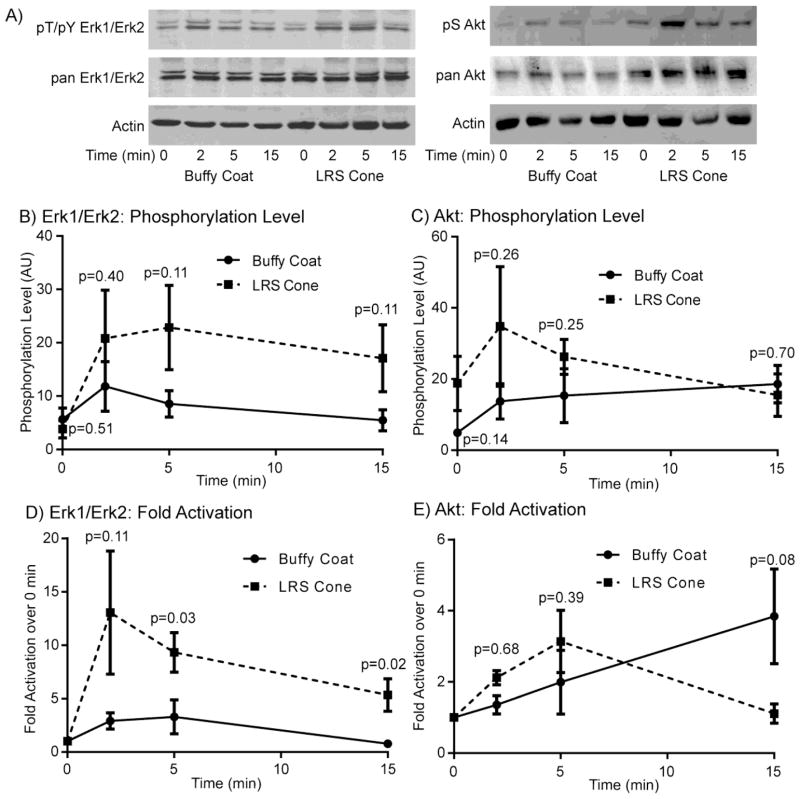
The previous experiments examining cytokine production suggest that TCR-mediated intracellular signaling could be enhanced in the LRS cone isolated T cells. To address this potential, activated T cells were generated using each isolation method and stimulated for various times with soluble stimulatory anti-CD3 and anti-CD4 antibodies. Phospho-specific antibodies were then used to measure the changes in ERK1/ERK2 or AKT phosphorylation after normalization to the expression of both actin and the individual protein. We have used this technique extensively to assess changes in the extent and timing of early signaling in human T cells [11–19]. ERK1/ERK2 and AKT activation are excellent markers for receptor-mediated signaling, since the function of these proteins in T cells has been linked to increased survival, proliferation and the induction of effector cytokines [20].
As shown in Figure 3A, the LRS cone isolated T cells had enhanced levels of ERK1/ERK2 and AKT phosphorylation compared to T cells isolated using buffy coats. When the levels of phosphorylation were normalized and compiled for multiple donors there was a consistent 3–4 fold increase in the amount of TCR-induced ERK1/ERK2 phosphorylation in the LRS isolated T cells, with little increase in the level of ERK activation in unstimulated T cells (Figure 3B). AKT phosphorylation at 2 and 5 minutes was also enhanced by 2–4 fold in T cells derived from LRS cones, with fewer differences at 15 minutes (Figure 3C). Strikingly, the level of basal AKT phosphorylation was consistently enhanced approximately 3–4 fold in LRS isolated T cells (Figure 3C). None of these values were statistically significant due to substantial donor to donor variation in the level of protein phosphorylation. When the fold activation over unstimulated cells was calculated, ERK1/ERK2 had a significantly enhanced fold activation in LRS derived T cells compared to buffy coat isolated T cells (Figure 3D). In contrast, AKT did not have a significant enhancement in fold activation over unstimulated cells in LRS derived T cells (Figure 3E), largely because the levels of AKT phosphorylation at the 0 minute timepoint was substantially different between the two isolation methods. Collectively, these results demonstrate that cells from LRS cone isolations have enhanced early TCR-mediated signaling compared to T cells isolated from standard buffy coats.
Figure 3.
Early TCR-mediated signaling is enhanced in T cells isolated from LRS cones. (A) T cells isolated using standard buffy coats or LRS cones were stimulated with anti-CD3 (10 μg/mL) and anti-CD4 (2 μg/mL) for various times. The phosphorylation of ERK1/ERK2 and AKT and the expression of ERK1/ERK2, AKT and actin were examined by immunoblotting. The results are a representative of six donors for each isolation method. The level phosphorylation of ERK1/ERK2 and AKT at each timepoint was normalized as described in the materials and methods. The relative phosphorylation levels for (B) ERK1/ERK2 and (C) AKT or the fold activation from the 0 timepoint for (D) ERK1/ERK2 and (E) AKT are shown as the average ± SEM of six donors for each isolation method with the outliers removed as described in the Materials and Methods. The p values for the statistical analysis of each point are shown on the graphs.
4. Discussion
The need for primary human T cells for research and clinical purposes has increased substantially in the last decade. The usage of primary human T cells will likely increase over the coming years due to the growing awareness of the limitations of human T cell lines [11, 21–24] and the increasing understanding that mouse models may poorly mimic human inflammatory disease and other disorders linked to T cell function [25, 26]. The current method for the acquisition of large numbers of human primary T cells is the isolation of PBMCs via standard buffy coat followed by the expansion of the T cells using stimulatory antibodies and recombinant IL-2. However, the drawback of buffy coat isolation is the limits on the number of cells due to the availability of donors and/or restrictions imposed by human subject research protocols. Isolation of PBMCs from LRS cones bypass these issues since the cones are a byproduct of blood donation, which occurs over 15,000,000 times per year in the USA alone according to the US Red Cross. Additionally, many research institutions are obtaining consent for the anonymous use of LRS cones in research studies, thus bypassing the requirement for IRB approval for individual investigators. Because of these benefits, LRS cone isolations have become increasingly popular as an efficient way of obtaining large numbers of PBMCs that can be further activated or purified to obtain human primary T cells [4].
Although T cells can be obtained from LRS cones, little is known about differences in T cell effector function between standard buffy coat and LRS cone isolations. Our studies are the first to address the question of whether different isolation methods result in functional alterations in T cells that are activated and expanded. This is a critical question since the majority of primary human T cells used for basic research have been expanded using non-specific mitogens, such as PHA or ConA, or stimulatory anti-CD3/CD28 antibodies in the presence of IL-2. Similarly, the vast majority of clinical applications for human primary T cells, including the expansion of tumor infiltrating lymphocytes or production of T cells with chimeric antigen receptors [1, 2], require activated and expanded human T cells. We observed that activated peripheral blood T cells derived from LRS cones had an increased percentage of activated T cells positive for the adhesion receptor CD44, a trend towards enhanced TCR-induced IL-2 and IFN-γ production, amplified donor-to-donor variability in IFN-γ production and heightened early TCR-mediated signaling through ERK1/ERK2 and AKT compared to T cells isolated by standard buffy coat. This suggests that isolation of T cells using LRS cones results in enhanced effector functions in primary human activated peripheral blood T cells.
The observation that there are alterations in the function of T cells isolated using different methods begs the question, what is the mechanism for these differences? One potential difference is the donors from the two methods. The donors for the standard buffy coat isolations in our study were undergraduate, graduate and laboratory personal that are generally between 20 to 40 years of age. In contrast, the donors from the LRS cones came from individuals that donated blood at the DeGowin Blood Center at the University of Iowa. These donors are generally older than 40 years old but for this study were restricted to donors under the age of 55. This suggests that alterations in the chronological age of populations of donors could be the mechanism for the observed differences between the isolation methods? Arguing against this possibility is the observation that T cells isolated from LRS cones, which are obtained from older donors, had enhanced early ERK1/ERK2 and AKT phosphorylation. Multiple studies have shown that T cells isolated from older individuals have reduced TCR-induced ERK1/ERK2 and AKT phosphorylation in CD4 T cells compared to T cells purified from younger donors [27], which is the exact opposite of what we observed. Similarly, T cells isolated from older individuals had reduced TCR-induced IL-2 but enhanced IFN-γ production compared to T cells isolated from younger donors, which again is different than the pattern we observed [28, 29]. These observations suggest that the age of donors is not the direct cause of differences in signaling and cytokine production between the two isolation methods.
If the age of the donors has no effect on the observed enhanced T cell function then the LRS cones themselves must be altering the function of the isolated T cells. The only difference in the isolation activated T cells obtained from PBMCs sourced from LRS cones or buffy coats was filtration using the cone. This suggests that interactions of the PBMCs and/or unactivated T cells with the filtration membrane of the LRS cones alters the subsequent function of activated T cells. In support of this model, Barbe and coworkers found that integrin molecules were important for the adhesion to filters, especially the β2-integrin [30]. T cells express lymphocyte function-associated antigen-1 (LFA-1), which is a member of the family of β2-integrins and regulates T cell function [31]. Henschler and coworkers observed that the adhesion of lymphocytes to LRS filters was dependent on calcium or the presence of platelets, which suggests integrin mediated interactions [32]. They also found that serum components that bind adhesion receptors, including fibronectin (VLA-4 integrin ligand) and hyaluronic acid (CD44 ligand), are also critical for lymphocyte adhesion [32]. Alexiou and coworkers observed that LRS filters selectively bound to white blood cells that expressed the CD11b integrin and CD62L, which are found on a subset of activated lymphocytes and naïve/central memory T cells, respectively [33]. In addition to interactions between the LRS cone matrix and adhesion receptors on the T cells, the LRS cones also concentrate T cells in specific regions of the filter. The majority of T cells are found in only five layers of the multi-layered LRS cones [32]. When the filter layers are analyzed by microcopy the vast majority of leukocytes and lymphocytes are found concentrated in discreet regions of the filter [32]. T cell to T cell contacts that occur in these regions of the filter are likely important because adhesion molecules, such as CD2, CD7, CD44 and integrins, are able to form interactions that induce functional changes in T cells. Thus, LRS cone filter material and the concentration of T cells on the filters could stimulate adhesion receptors that would modulate the function of subsequently activated T cells, leading to the enhanced function of T cells from LRS cones compared to cells isolated from buffy coats.
5. Conclusions
In conclusion, we have shown that activated T cells produced from LRS cone isolations have altered TCR-induced functions compared to T cells produced using standard buffy coats. The two methods may in fact model T cells from different anatomical regions. Buffy coat T cells are isolated from peripheral blood and retain similar conditions to what is found in the blood. In contrast, LRS cones provide adhesion receptor and cell-to-cell contacts that are similar to what T cells receive in the secondary lymphoid tissues and sites of inflammation. Ultimately, LRS cones are an efficient and cost-effective source of T cells for clinical and research applications, but these cells are functionally distinct from T cells isolated using standard buffy coat purification.
Acknowledgments
We thank the laboratory of Jessica Moreland for providing the PBMCs isolated from the buffy coat. We also thank Aldo Vacaflores, Mahmood Bilal and Michael Zhang for careful reading of the manuscript. These studies were supported by R01 CA136729 from the National Institutes of Health to J.C.D.H.. M.M.T. conceived, performed and analyzed experiments and wrote the manuscript. J.C.D.H. conceived and analyzed experiments and wrote the manuscript.
Abbreviations
- LRS
leukocyte reduction system
- PVDF
polyvinylidene difloride
- SEB
Staphylococcal enterotoxin B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shi H, Liu L, Wang Z. Improving the efficacy and safety of engineered T cell therapy for cancer. Cancer Lett. 2013;328:191–197. doi: 10.1016/j.canlet.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Park TS, Rosenberg SA, Morgan RA. Treating cancer with genetically engineered T cells. Trends Biotechnol. 2011;29:550–557. doi: 10.1016/j.tibtech.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebner S, Neyer S, Hofer S, Nussbaumer W, Romani N, Heufler C. Generation of large numbers of human dendritic cells from whole blood passaged through leukocyte removal filters: an alternative to standard buffy coats. J Immunol Methods. 2001;252:93–104. doi: 10.1016/s0022-1759(01)00337-4. [DOI] [PubMed] [Google Scholar]
- 4.Meyer TP, Zehnter I, Hofmann B, Zaisserer J, Burkhart J, Rapp S, Weinauer F, Schmitz J, Illert WE. Filter Buffy Coats (FBC): a source of peripheral blood leukocytes recovered from leukocyte depletion filters. J Immunol Methods. 2005;307:150–166. doi: 10.1016/j.jim.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Neron S, Dussault N, Racine C. Whole-blood leukoreduction filters are a source for cryopreserved cells for phenotypic and functional investigations on peripheral blood lymphocytes. Transfusion. 2006;46:537–544. doi: 10.1111/j.1537-2995.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 6.Pfeiffer IA, Zinser E, Strasser E, Stein MF, Dorrie J, Schaft N, Steinkasserer A, Knippertz I. Leukoreduction system chambers are an efficient, valid, and economic source of functional monocyte-derived dendritic cells and lymphocytes. Immunobiology. 2013;218:1392–1401. doi: 10.1016/j.imbio.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Dietz AB, Bulur PA, Emery RL, Winters JL, Epps DE, Zubair AC, Vuk-Pavlovic S. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006;46:2083–2089. doi: 10.1111/j.1537-2995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 8.Houtman JC, Houghtling RA, Barda-Saad M, Toda Y, Samelson LE. Early phosphorylation kinetics of proteins involved in proximal TCR-mediated signaling pathways. J Immunol. 2005;175:2449–2458. doi: 10.4049/jimmunol.175.4.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawilowsky SS, Fermat Schubert. Einstein, and Behrens-Fisher: The Probable Difference Between Two Means When σ12≠σ22. Journal of Modern Applied Statistical Methods. 2002;1:461–472. [Google Scholar]
- 11.Bartelt RR, Cruz-Orcutt N, Collins M, Houtman JC. Comparison of T cell receptor-induced proximal signaling and downstream functions in immortalized and primary T cells. PLoS ONE. 2009;4:e5430. doi: 10.1371/journal.pone.0005430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman NM, Bilal MY, Cruz-Orcutt N, Knudson C, Madinaveitia S, Light J, Houtman JC. Distinct signaling pathways regulate TLR2 co-stimulatory function in human T cells. Cell Signal. 2013;25:639–650. doi: 10.1016/j.cellsig.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman NM, Connolly SF, Reinl EL, Houtman JC. Focal adhesion kinase negatively regulates lck function downstream of the T cell antigen receptor. J Immunol. 2013;191:6208–6221. doi: 10.4049/jimmunol.1301587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins M, Bartelt RR, Houtman JC. T cell receptor activation leads to two distinct phases of Pyk2 activation and actin cytoskeletal rearrangement in human T cells. Mol Immunol. 2010;47:1665–1674. doi: 10.1016/j.molimm.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Collins M, Tremblay M, Chapman N, Curtiss M, Rothman PB, Houtman JC. The T cell receptor-mediated phosphorylation of Pyk2 tyrosines 402 and 580 occurs via a distinct mechanism than other receptor systems. J Leukoc Biol. 2010;87:691–701. doi: 10.1189/jlb.0409227. [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Orcutt N, Houtman JCD. PI3 kinase function is vital for the function but not formation of LAT-mediated signaling complexes. Mol Immunol. 2009;46:2274–2283. doi: 10.1016/j.molimm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Orcutt N, Vacaflores A, Connolly SF, Bunnell SC, Houtman JC. Activated PLC-gamma1 is catalytically induced at LAT but activated PLC-gamma1 is localized at both LAT- and TCR-containing complexes. Cell Signal. 2014 doi: 10.1016/j.cellsig.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houtman JCD, Houghtling RA, Barda-Saad M, Toda Y, Samelson LE. Early phosphorylation kinetics of proteins involved in proximal TCR-mediated signaling pathways. J Immunol. 2005;175:2449–2458. doi: 10.4049/jimmunol.175.4.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay MM, Bilal MY, Houtman JC. Prior TLR5 induction in human T cells results in a transient potentiation of subsequent TCR-induced cytokine production. Mol Immunol. 2014;57:161–170. doi: 10.1016/j.molimm.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeburn RW, Wright KL, Burgess SJ, Astoul E, Cantrell DA, Ward SG. Evidence that SHIP-1 contributes to phosphatidylinositol 3,4,5-trisphosphate metabolism in T lymphocytes and can regulate novel phosphoinositide 3-kinase effectors. J Immunol. 2002;169:5441–5450. doi: 10.4049/jimmunol.169.10.5441. [DOI] [PubMed] [Google Scholar]
- 22.Seminario M-C, Precht P, Bunnell SC, Warren SE, Morris CM, Taub D, Wange RL. PTEN permits acute increases in D3-phosphoinositide levels following TCR stimulation but inhibits distal signaling events by reducing the basal activtiy of Akt. Eur J Immunol. 2004;34 doi: 10.1002/eji.200425206. In Press. [DOI] [PubMed] [Google Scholar]
- 23.Shan X, Czar MJ, Bunnell SC, Liu P, Liu Y, Schwartzberg PL, Wange RL. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol Cell Biol. 2000;20:6945–6957. doi: 10.1128/mcb.20.18.6945-6957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finger LR, Huebner K, Cannizzaro LA, McLeod K, Nowell PC, Croce CM. Chromosomal translocation in T-cell leukemia line HUT 78 results in a MYC fusion transcript. Proc Natl Acad Sci U S A. 1988;85:9158–9162. doi: 10.1073/pnas.85.23.9158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leist M, Hartung T. Inflammatory findings on species extrapolations: humans are definitely no 70-kg mice. Arch Toxicol. 2013;87:563–567. doi: 10.1007/s00204-013-1038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larbi A, Pawelec G, Wong SC, Goldeck D, Tai JJ, Fulop T. Impact of age on T cell signaling: a general defect or specific alterations? Ageing Res Rev. 2011;10:370–378. doi: 10.1016/j.arr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Larbi A, Dupuis G, Khalil A, Douziech N, Fortin C, Fulop T., Jr Differential role of lipid rafts in the functions of CD4+ and CD8+ human T lymphocytes with aging. Cell Signal. 2006;18:1017–1030. doi: 10.1016/j.cellsig.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Schindowski K, Frohlich L, Maurer K, Muller WE, Eckert A. Age-related impairment of human T lymphocytes’ activation: specific differences between CD4(+) and CD8(+) subsets. Mech Ageing Dev. 2002;123:375–390. doi: 10.1016/s0047-6374(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 30.Barbe LL, Boval BM, Wautier MP, Wautier JL. An in vitro system for testing leucocyte and leukaemic cell line adhesion to synthetic fibres. Br J Haematol. 2001;115:664–671. doi: 10.1046/j.1365-2141.2001.03137.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Wang H. Integrin signalling and function in immune cells. Immunology. 2012;135:268–275. doi: 10.1111/j.1365-2567.2011.03549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henschler R, Ruster B, Steimle A, Hansmann HL, Walker W, Montag T, Seifried E. Analysis of leukocyte binding to depletion filters: role of passive binding, interaction with platelets, and plasma components. Ann Hematol. 2005;84:538–544. doi: 10.1007/s00277-004-0994-0. [DOI] [PubMed] [Google Scholar]
- 33.Alexiou C, Sheppard S, Tang A, Rengarajan A, Smith D, Haw M, Gibbs R. Leukocytes-depleting filters preferentially remove activated leukocytes and reduce the expression of surface adhesion molecules during the simulated extracorporeal circulation of human blood. Asaio J. 2006;52:438–444. doi: 10.1097/01.mat.0000225894.61493.2f. [DOI] [PubMed] [Google Scholar]