Figure 8.
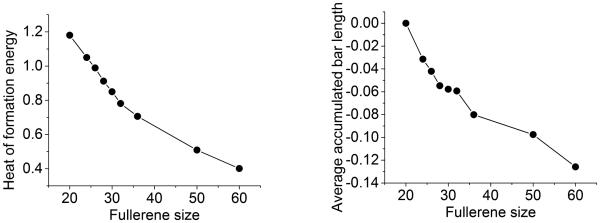
Comparison between the heat of formation energies computed using a quantum theory56 (left chart) and average accumulated bar length (right chart) for fullerenes. The units for the heat of formation energy and average accumulated bar length are eV/atom and Å/atom, respectively Although the profile of average accumulated bar length of fullerenes does not perfectly match the fullerene energy profile, they bear a close resemblance in their basic characteristics.
