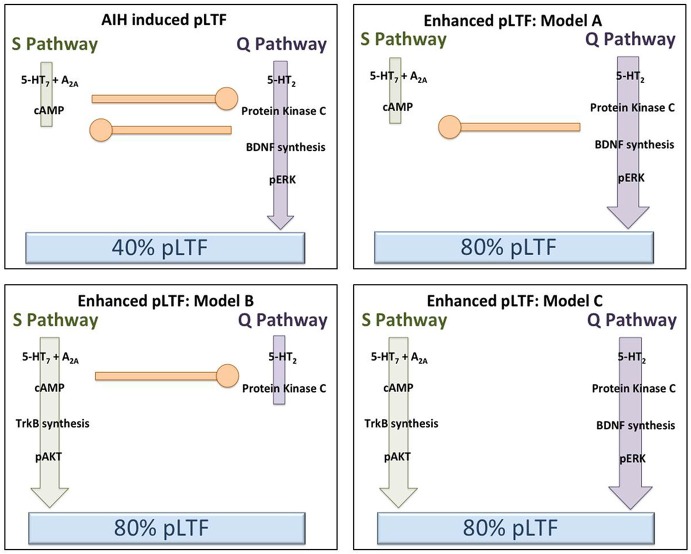
Figure 3.
Hypothetical models to explain pLTF metaplasticity (enhanced pLTF) after repetitive acute intermittent hypoxia (rAIH) preconditioning. In the upper left panel, our working model of Q and S pathway contributions to pLTF in normal rats is depicted (no preconditioning). Moderate AIH normally elicits ~40% pLTF, largely via dominant contributions from the Q pathway, with concurrent restraint from sub-threshold S pathway activation (i.e., cross-talk inhibition). In the remaining panels, rAIH preconditioning enhances AIH-induced pLTF, reaching ~80% facilitation. In the upper right (Model A), we illustrate the possibility that rAIH preconditioning enhances pLTF by enhancing the Q pathway to pLTF. This could be achieved by amplifying the Q pathway, or by removing inhibition from the S pathway (while leaving Q to S inhibition intact). For example, pLTF is doubled in normal rats when cross-talk inhibition from the S pathway is reduced from cervical spinal inhibition of A2A receptors, 5-HT7 receptors or PKA (see text for references). In either case, the Q pathway remains the dominant pathway to pLTF in this scenario, similar to enhanced pLTF following chronic cervical dorsal rhizotomy (CDR) or during end-stage amyotrophic lateral sclerosis (ALS) (see text for references). In the lower left panel (Model B), we illustrate the possibility that pLTF following rAIH preconditioning arises from a reversal to dominant S pathway contributions to pLTF vs. Q pathway-dependent pLTF found in normal rats. There is little available evidence to support this possibility; however, the precedent is provided by the greater S pathway-dependent pLTF resulting from severe AIH protocols (Nichols et al., 2012). In the lower right panel (Model C), we illustrate the possibility that rAIH preconditioning somehow eliminates cross-talk inhibition and uncouples the Q and S pathways to pMF; thus, each pathway is now able to contribute to an enhanced pLTF. This possibility is supported by available evidence concerning mechanisms of enhanced pLTF after CIH pre-conditioning, where both 5-HT2 and 5-HT7 receptors appear to make independent contributions (see text).