Figure 1.
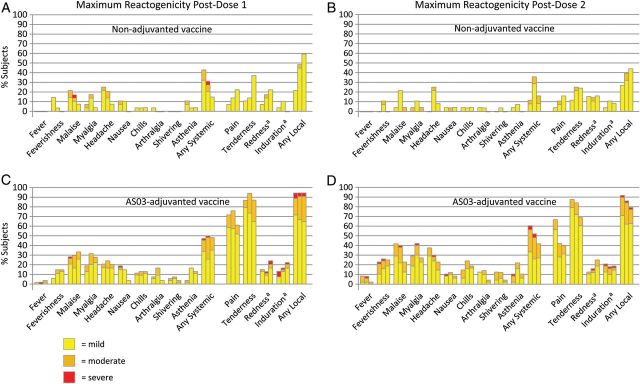
The percentage of subjects who experienced solicited adverse events, by maximum reactogenicity, during the 7 days after receipt of the first dose (A and C) or the second dose (B and D), according to vaccine dosage (3.75, 7.5, and 15 µg) and whether nonadjuvanted (A and B) or AS03-adjuvanted (C and D). aThe widest diameter was measured and graded as follows: small (mild) <20 mm, medium (moderate) 20–50 mm, and large (severe) >50 mm.
