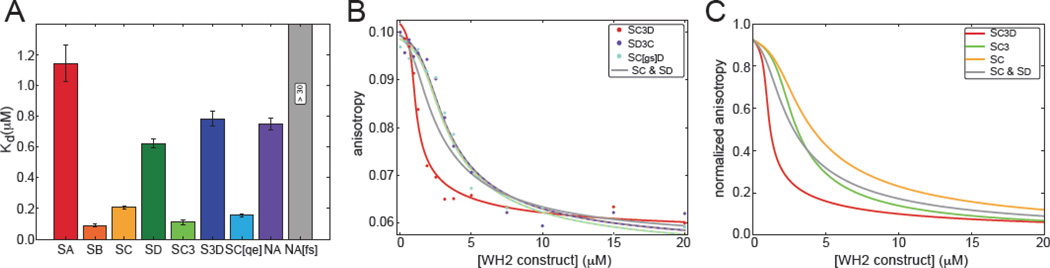
Figure 3.
Cooperative binding by tandem WH2 domains depends on domain order. (A) The reported Kd values of WH2 constructs bound to latB-actin are the mean of three independent trials of competition fluorescence anisotropy with Sd-AlexaFluor488 (Figure S3). Na[fs] binds too weakly to determine its affinity for actin monomers. Error bars represent one standard deviation. (B) Representative competition fluorescence anisotropy with Sd-AlexaFluor488 and latB-actin as a function of added Sc3d (red circles), Sd3c (purple circles) or Sc[gs]d (aqua circles). Data are fit with a two-site equilibrium binding model. Regressions are in the same color as the data set. The dashed lines represent modeling assuming the Kd for a second actin monomer is infinite for Sd3c or Sc[gs]d. The grey trace is a theoretical plot based on measured affinities for Sc and Sd, with independent binding. (C) Anisotropy curves of Sc3d, Sc3 and Sc based on measured affinities were normalized for comparison. The grey trace is a theoretical plot based on measured affinities for Sc and Sd, with independent binding.