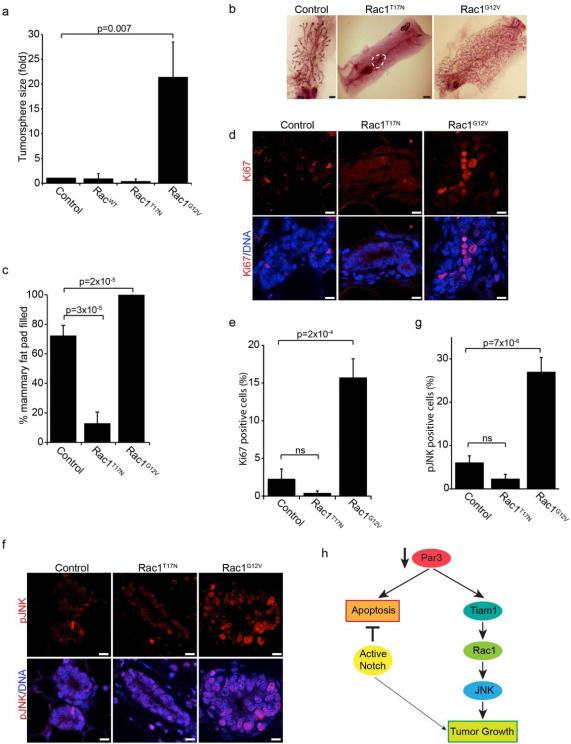
Figure 4. Rac1/JNK signaling regulates mammary epithelial proliferation.
(a) Quantification of tumorsphere sizes of MECs expressing GFP (control), wild-type (Rac1WT), dominant negative (Rac1T17N) or constitutively active (Rac1G12V) Rac1. (b) Mammary epithelial cells were transduced with control (GFP alone), Rac1T17N, or Rac1G12V lentivirus and then transplanted into a cleared mammary fat pad and allowed to regrow for 8-weeks. (c) Quantification of the percentage of the fat pad filled from (b), n=4. (d) Tissue sections of control (GFP), Rac1T17N, and Rac1G12V mammary glands were immunostained for Ki67. (e) Quantification of cells with nuclear Ki67 from (d). All ducts from 2 tissue sections for each of 3 glands were measured. (f) Tissue sections of control (GFP), Rac1T17N, and Rac1G12V mammary glands were stained for phospho-JNK. (g) Quantification of cells with nuclear phospho-JNK from (f). (h) Model showing that loss of Par3 in the normal mammary epithelium activates both apoptotic and proliferative responses. In the presence of an anti-apoptotic factor (i.e. Notch oncogene), the apoptotic response caused by a loss of Par3 is suppressed and a Tiam1/Rac1/JNK mediated proliferative response is revealed. p-values were calculated using the ANOVA with Tukey HSD post-hoc test. Error bars represent sd (a) or sem (c, e, and (g). Scale bars = 2mm (b) or 10 μm (d and f).