Abstract
Francisella tularensis causes disease (tularemia) in a large number of mammals, including man. We previously demonstrated enhanced efficacy of conventional antibiotic therapy for tularemia by postexposure passive transfer of immune sera developed against a F. tularensis LVS membrane protein fraction (MPF). However, the protein composition of this immunogenic fraction was not defined. Proteomic approaches were applied to define the protein composition and identify the immunogens of MPF. MPF consisted of at least 299 proteins and 2-D Western blot analyses using sera from MPF-immunized and F. tularensis LVS-vaccinated mice coupled to liquid chromatography–tandem mass spectrometry identified 24 immunoreactive protein spots containing 45 proteins. A reverse vaccinology approach that applied labeling of F. tularensis LVS surface proteins and bioinformatics was used to reduce the complexity of potential target immunogens. Bioinformatics analyses of the immunoreactive proteins reduced the number of immunogen targets to 32. Direct surface labeling of F. tularensis LVS resulted in the identification of 31 surface proteins. However, only 13 of these were reactive with MPF and/or F. tularensis LVS immune sera. Collectively, this use of orthogonal proteomic approaches reduced the complexity of potential immunogens in MPF by 96% and allowed for prioritization of target immunogens for antibody-based immunotherapies against tularemia.
Keywords: Francisella tularensis, surface proteome, membrane proteome, adaptive immunity, antibodies
1. Introduction
Francisella tularensis, the etiological agent of tularemia, causes an acute infection with several clinical manifestations, including a pneumonic presentation that is the most severe and ulceroglandular disease that is the most common.1,2F. tularensis subsp. tularensis (type A) and F. tularensis subsp. holarctica (type B) both cause disease in humans, but type-B infections are rarely fatal. In contrast, pneumonic disease caused by F. tularensis subsp. tularensis results in mortalities ranging between 30 and 60% if left untreated.3F. tularensis infections are treatable by a wide array of antibiotics including gentamicin, but these need to be administered in a timely manner to avoid increased chance of relapse.3 The importance of the humoral response against F. tularensis to control and clear infection is also recognized. Foshay et al. showed that passive transfer of F. tularensis immune sera provided prophylactic protection in humans.4 Similarly, Drabick et al. demonstrated that passive transfer of immune sera protected mice against a lethal high dose challenge with F. tularensis subsp. holarctica live vaccine strain (LVS), and this protection was abrogated by preabsorption of the serum with a F. tularensis LVS lysate, thus implicating antibodies as the protective component.5 Passively transferred F. tularensis LVS immune serum also decreased the duration and severity of a type A infection in rats as well as reduced systemic bacterial burden to the liver and spleen.6
Membrane components of F. tularensis have shown protective efficacy in prophylactic and postexposure therapeutic models of tularemia.7−9 Ireland et al. demonstrated the protective effects of adjuvant complexed with a membrane protein fraction (MPF) when administered prophylactically 3 days prior to a virulent F. tularensis SCHU S4 challenge in mice.8 Huntley et al. isolated outer membrane proteins and lipopolysaccharide (LPS) from F. tularensis LVS and found that vaccination with these provided 50 and 15% increase in survival, respectively, in mice challenged with F. tularensis SCHU S4.9 While LPS provided a degree of protection in immunized mice, passive transfer of F. tularensis LVS LPS immune sera provided little to no protection against a F. tularensis SCHU S4 challenge.10,11
To evaluate membrane-based immunotherapeutic methods that enhance chemotherapy, we created a murine model of tularemia treated with a subtherapeutic regimen of gentamicin. Using this model, it was demonstrated that postexposure vaccination with the MPF of F. tularensis LVS provided full protection in the presence of a subtherapeutic dose of gentamicin against a type A F. tularensis strain SCHU S4 infection (100% survival at day 40 of infection).7 Moreover, the passive transfer of the MPF immune sera restored complete efficacy to the suboptimal gentamicin regime, indicating antibodies as the protective component in this model. The protective immune sera from our postexposure subtherapeutic gentamicin and MPF vaccination murine model showed high IgM, IgG3, and IgG2a titers with the IgM response directed at LPS and the IgG response directed toward membrane proteins.7 Additionally, these mice showed a reduced severity of disease once the adaptive immune response initiated the production of high IgG titers, indicating that MPF proteins were important immunogenic components of MPF. However, the protein targets of these protective antibodies were not defined.
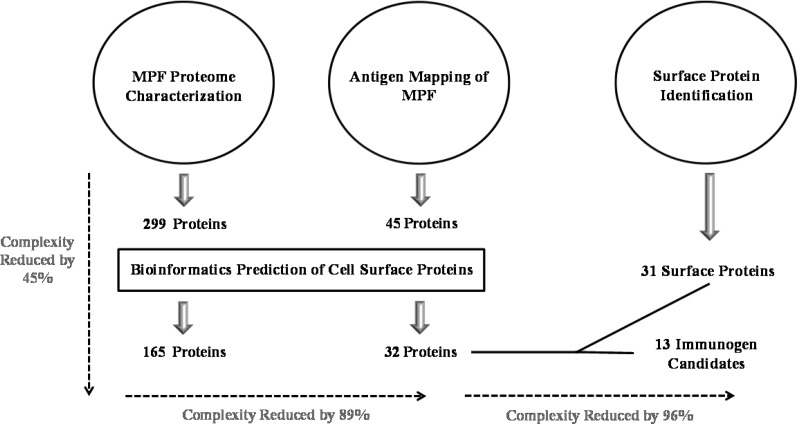
In the present study, we characterized the MPF proteome and applied the principles of reverse vaccinology to identify the likely immunogens of MPF (Figure 1). The concept behind reverse vaccinology is that successful protein-based bacterial immunotherapies are formulated with surface-exposed or -secreted bacterial proteins. Reverse vaccinology utilizes orthogonal high-throughput bioinformatics and proteomic pipelines to identify surface proteins, dramatically reducing the number of candidate immunogens to test in animal models.12,13 The immunogen signatures profiled in this study included bioinformatic predictions of membrane and surface localization and secretion, immunoreactivity to corresponding murine immune sera (MPF immunized and F. tularensis LVS vaccinated), and experimental validation of cell surface localization. The MPF consisted of at least 299 proteins, of which 45 immunoreactive proteins were identified. Of the immunoreactive proteins, 13 localized to the bacterial cell surface, suggesting they are the immunogenic protein components of the F. tularensis LVS MPF.
Figure 1.
Schematic of the experimental workflow used to identify F. tularensis LVS MPF immunogens.
2. Materials and Methods
2.1. Bacteria, Culture Conditions, and MPF Isolation
F. tularensis LVS was provided by Dr. Jeannine Petersen (Centers for Disease Control and Prevention, Fort Collins, CO). For identification of surface proteins, F. tularensis LVS was grown on CHAB medium (cysteine heart agar supplemented with 9% chocolatized sheep blood) for 48 h at 37 °C. Cells were collected by scraping and suspended in phosphate-buffered saline (PBS) (pH 7.4) for surface labeling. F. tularensis LVS, used for the generation of MPF, was grown on CHAB plates for 48 h at 37 °C and subcultured in 50 mL of modified Mueller Hinton (MMH) broth supplemented with 2% Isovitalex (BD, Sparks, MD), 0.1% glucose, and 0.025% ferric pyrophosphate with shaking (150 rpm) at 37 °C for 12 h, followed by inoculation of 1 L of MMH broth. After 12 h, cells were collected by centrifugation at 3000g and washed in PBS, pelleted by centrifugation, and stored at −80 °C. The MPF of F. tularensis strain LVS was prepared, quantified, and tested for foreign endotoxin contamination as described previously.7
2.2. Mice
Specific pathogen-free, 6–8 week old BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were provided with sterile water and food ad libitum, and all research involving animals was conducted in accordance with Animal Care and Use guidelines and approved by the Animal Care and Use Committees at Colorado State University and Rocky Mountain Laboratories.
2.3. Immunization of Mice
For generation of MPF immune sera, mice were given 10 μg of MPF diluted in 5% dextrose water (D5W) and intraperitoneal (i.p.). Mice were immunized twice, 4 days apart, and serum was drawn via cardiac puncture 7 days after the first injection. Anti-MPF antibody titers were confirmed via ELISA with naïve serum used as a control.7
Mice were immunized twice (2 weeks apart) subcutaneously (s.c.) with F. tularensis LVS diluted in PBS (200 CFU in 200 μL). The CFU concentration of the inoculum was confirmed by plating on CHAB medium and enumerating colonies after 48 h. Unvaccinated mice served as negative sera controls. All mice were bled via the tail vein for serum collection 12 days after immunization.
2.4. Surface Labeling of Proteins and Label Localization
Suspensions of F. tularensis LVS were pelleted by centrifugation at 4500g, washed three times in PBS, and adjusted to an A600 OD of 0.15 to 0.19 in PBS. An aliquot (80 μL) of EZ-Link Sulfo-NHS-LC-Biotin (LC-Biotin) (Thermo/Pierce, Rockford, IL) at 6.6 mg/mL was added per 1 mL of the cell suspension and incubated at room temperature for 1 h with gentle rocking. The cells were collected by centrifugation and washed once in Tris-buffered saline (TBS) (pH 7.4), and twice in PBS. The final biotin-labeled cell pellet was suspended in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (0.3 M Tris, 50% glycerol, 10% sodium dodecyl sulfate, 25% β-mercaptoethanol, and trace bromophenol blue) for analysis by SDS-PAGE or in 1% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate hydrate (CHAPS) buffer (1% CHAPS in PBS) for affinity purification of labeled proteins.
F. tularensis LVS whole cell lysates (WCLs) from cells grown on CHAB were prepared by suspending cells in breaking buffer [PBS, pH 7.4, 60 μg of DNase (Sigma, St. Louis, MO), 60 μg of RNase (Sigma), 50 μg of lysozyme (Sigma), and one Complete Protease Inhibitor tablet (Roche Applied Sciences, Indianapolis, IN) per 50 mL of buffer]. Cells were placed on ice and lysed by nine repetitions of pulsed sonication using a 4710 series ultrasonic homogenizer (Cole and Palmer Instrument Company, Vernon Hills, IL) employing the following instrument parameters: 50% duty, output-5, 60 s pulses, and 60 s pauses. Unbroken cells were removed from the lysate by centrifugation at 4500g, 4 °C, for 20 min. Lysates were labeled with LC-Biotin as described for whole cells.
2.5. Biotinylated Protein Purification
Surface-labeled proteins were extracted for affinity purification by suspending cells in 1% CHAPS buffer for 15 min at 120 °C with intermittent vortexing. Extracts were added to immobilized streptavidin resin (Thermo/Pierce) pre-equilibrated in PBS and incubated overnight at 4 °C with gentle shaking. The resin was washed with 10 vol of 1% CHAPS buffer, followed by 10 vol of PBS. Biotinylated proteins were eluted from the resin in SDS-PAGE sample buffer at 120 °C for 10 min.
2.6. Polyacrylamide Gel Electrophoresis
2D-PAGE was performed with 100 μg aliquots of MPF. Sample preparation was performed with the ReadyPrep 2-D Cleanup Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. The resulting protein pellet was air-dried and suspended in 22.5 μL of 50 mM Tris (pH 7.1), 8 M urea, 2 M thiourea, 100 mM dithiothreitol (DTT), 4% (w/v) CHAPS, 1% (w/v) ASB-14, 0.7% pH 4–7 ampholytes, and 0.3% pH 3–10 ampholytes. The proteins were sonicated and incubated for 4 h at room temperature. Samples were diluted with a 130 μL of solution of 8 M urea, 2 M thiourea, 20 mM DTT, 4% (w/v) CHAPS, 1% (w/v) ASB-14, 0.7% pH 4–7 ampholytes, and 0.3% pH 3–10 ampholytes and centrifuged for 30 min at 13 000g to remove any insoluble material. Supernatants (130 μL) were applied to an Immobiline dry strip (GE Healthcare Life Sciences, Piscataway, NJ) with either pH gradients of 4–7 or 6–11 following the manufacturer’s instructions.
Isoelectric focusing (IEF) was performed using the Multiphore II unit (GE Healthcare Life Sciences) at 50, 100, 150, 200, 250, and 300 V sequentially for 6 min, followed by 500 V for 12 min and 3000 V for 5 h. The focused Immobiline strips were rinsed in ultrapure H2O and incubated in 0.375 M Tris (pH 7.1), 6 M urea, 2% SDS, 30% glycerol, 1% DTT, and trace bromophenol blue for 15 min at room temperature. Immobiline strips were again rinsed in ultrapure H2O and incubated in 0.375 M Tris (pH 7.1), 6 M urea, 2% SDS, 30% glycerol, 2.5% iodoacetamide, and trace bromophenol blue before a final rinsing in ultrapure H2O. Proteins were resolved in the second dimension by SDS-PAGE.
Aliquots of labeled proteins and MPF were applied to SDS-PAGE using a 4–12% Bis-Tris SDS-polyacrylamide gels (Life Technologies, Carlsbad, CA) under denaturing conditions.14
Detection and staining of protein in polyacrylamide gels was accomplished with the Pierce Silver Stain Kit (Thermo/Pierce) or SimplyBlue SafeStain (Life Technologies) according to the respective manufacturer’s protocols. Gel images were converted to digital data using a HP Scanjet 4850 photo scanner (Hewlett-Packard Company, Palo Alto, CA).
2.7. Western Blotting
Biotinylated surface proteins and MPF resolved by SDS-PAGE were transferred to nitrocellulose membranes,15 incubated in TBS containing 0.1% Tween 20 and 5% nonfat milk and washed in TBS containing 0.1% Tween 20 (TBST). The nitrocellulose membranes were probed with pooled sera (1:200) from vaccinated or control mice or with antibiotin antibody conjugated to horseradish peroxidase (HRP) (Cell Signaling Technology, Danvers, MA) and subsequently washed in TBST. Immune sera Western blots were incubated with alkaline-phosphatase-conjugated goat-antimouse IgG (1:5000) to detect primary antibodies and washed with TBST, and antibody-reactive proteins were detected by BCIP/NBT SigmaFAST tablets (Sigma). Western blot images were digitized using a HP Scanjet 4850 photo scanner (Hewlett-Packard Company, Palo Alto, CA). Membranes receiving the antibiotin-HRP as the primary probe were developed with LumiGLO (Cell Signaling Technology) using CL-Exposure film (Thermo/Pierce). Digitized images of antibiotin Western blots were analyzed with Imagequant TL software (GE Health Care Life Sciences), and the number of reactive protein bands in each sample was determined based on densitometry.
2.8. Fractionation of MPF Tryptic Peptides
An aliquot (400 μg) of MPF suspended in 50 μL of 0.2 M ammonium bicarbonate was digested with 10 μg modified trypsin (Roche Applied Science) at 37 °C for 4 h, followed by overnight digestion with an additional 10 μg of trypsin. Samples were dried under vacuum, reconstituted in H2O, and dried three times before suspension in 120 μL of 3% acetonitrile (ACN) containing 0.1% acetic acid. Insoluble material was removed from the sample by centrifugation at 16 000g for 10 min.
Peptides were separated by strong cation exchange (SCX) chromatography using an Alliance 2695 HPLC (Waters, Milford, MA) coupled to a PolyLC polysulfethyl A column (4.6 mm × 100 mm) (The Nest Group, Southboro, MA) with an increasing linear gradient of KCl (0 to 500 mM) in 10 mM KH2PO4, 25% ACN and a flow rate of 1 mL/min. A total of 12 fractions were collected and dried under vacuum. The fractions were reconstituted in 100 μL of 0.1% trifluoroacetic acid and desalted using OMIX C18 tips according to the manufacturer’s instructions (Varian, Palo Alto, CA). Desalted samples were dried under vacuum and suspended in 11 μL of 3% ACN, 0.1% formic acid. Samples were sonicated for 5 min, followed by centrifugation for 10 min at 16 000g and transferred to an autosampler vial for LC–MS/MS analyses as described below.
2.9. Protein Identification by Liquid Chromatography–Mass Spectrometry
Affinity purified surface proteins and MPF proteins resolved by SDS-PAGE were subjected to in-gel proteolytic digestion with trypsin or chymotrypsin as described previously.16
Peptides from affinity purified surface proteins were applied to capillary C18 reverse-phase columns (Zorbax 300SB C18, 3.5 μm particle size, 0.3 × 150 mm, Agilent Technologies, Santa Clara, CA) and eluted with an increasing linear gradient of ACN in 0.1% formic acid at a flow rate of 5 μL/min using an Agilent 1100 capillary HPLC solvent delivery system. Effluent was introduced directly into a ThermoFinnigan (San Jose, CA) LTQ mass spectrometer (LTQ) operated with Xcalibur software ver. 2.0 SR2. For ionization and fragmentation, the mass spectrometer was configured with an electrospray voltage of 4 kV, a N2 sheath gas flow of 15, a capillary temperature of 200 °C, and a normalized collision energy of 35%. The top five most intense ions from the full MS scan (m/z range of 400 to 2000 Da) were selected for MS/MS (a maximum of two times per precursor). Selected precursor ions were then placed on the dynamic exclusion list for one min.
LC–MS/MS of tryptic digests of 2D-PAGE protein spots and MPF peptides separated by SCX was achieved using an Agilent 1200 nano flow LC system coupled via a Chip Cube interface to an Agilent 6520 quadrapole time-of-flight mass spectrometer (Q-TOF) operated with MassHunter Workstation Software ver. B.06.00 (Agilent). Peptides were resolved with an increasing linear gradient (36 min, 10 to 90%) of ACN applied to 43 mm 300 Å C18 chip in-line with a 40 nL trap column (ProtID-Chip-43) (Agilent). Peptides were eluted directly into the mass spectrometer at a rate of 0.5 μL/min and MS spectra were collected in positive ion mode over a m/z range of 250 to 2400 Da. MS/MS data were collected using a ramped collision energy with a slope of 3.7 and an offset of 2.5. Three precursor ions were selected for each MS/MS cycle. These analyses were performed in the BioMolecular Analysis Core at Colorado State University (http://www.rmrce.colostate.edu/pages/scientific-cores/BioMolecular-Analysis).
Tryptic digests of selected low-abundance 2D-PAGE protein spots were analyzed using a Thermo Scientific Orbitrap Velos (Orbitrap) operated with Xcalibur ver.2.2 SP1 software (Thermo Scientific). Peptides were applied to an in-line enrichment column (Thermo scientific EASY-Column, 2 cm, ID 100 μm, 5 μm, C18-A1), and subsequent chromatographic separation was performed on a reversed-phase nanospray column (Thermo Scientific EASY-Column 10 cm, ID 75 μm, 3 μm, C18-A2) using an increasing linear gradient (10 to 30%) of ACN at a flow rate of 0.4 μL/min. Peptides were eluted directly into the mass spectrometer, and data-dependent spectral acquisition was performed over a m/z range of 400 to 2000 Da at a normalized collision energy of 35%. The instrument was operated in Orbitrap-LTQ mode, where precursor measurements were acquired at 60 000 resolution, and MS/MS spectra were acquired in the LTQ ion trap using dynamic exclusion of two MS/MS spectra per precursor ion over 30 s and an exclusion duration of 90 s. These analyses were performed in the Proteomics and Metabolomics Facility at Colorado State University (www.pmf.colostate.edu).
2.10. Database Searching
Q-TOF derived MS/MS data were extracted using MassHunter Workstation software. LTQ and Orbitrap data were extracted using MSConvert (Proteowizard, http://proteowizard.sourceforge.net). All MS/MS data were searched using Mascot (Matrix Science, London, U.K.; ver. 2.3.02) and X! Tandem (The Global Proteome Machine Organization, www.thegpm.org; ver. CYCLONE (2010.12.01.1)). Mascot and X! Tandem were set to search the NCBInr_011014 database (F. tularensis, 7532 entries). MS/MS data acquired from the Q-TOF were searched with a fragment ion mass tolerance of 0.01 Da and a parent ion tolerance of 20 ppm. LTQ data were searched with a fragment ion mass tolerance of 1.0 Da and a parent ion tolerance of 2.5 Da. Orbitrap-derived MS/MS data were searched with a fragment ion mass tolerance of 0.8 Da and a parent ion tolerance of 20 ppm. For all data, variable modifications of glutamic acid to pyroglutamic acid of the N-terminus, ammonia-loss of the N-terminus, glutamine to pyroglutamic acid of the N-terminus, and oxidation of methionine were considered. For affinity-purified surface proteins and for protein spots, biotinylation of lysine (339.16 Da) and carbamidomethylation of cysteine, respectively, were also considered as variable modifications.
Scaffold ver. 4.3.2 (Proteome Software, Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at >90% probability by the Peptide Prophet algorithm with Scaffold delta-mass correction.17 Protein identifications were accepted if they could be established at >99% probability and contained at least two identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm.18 Proteins that contained similar peptides and could not be differentiated based on MS/MS analyses alone were grouped to satisfy the principles of parsimony. For protein spots or fractions where biological replicates were obtained, data files were combined using the “mudpit” function to generate a composite list of the proteins identified in the specific sample. Protein and peptide false discovery rates (FDRs) were calculated using the assigned probabilities estimated from the results of Peptide and Protein Prophet. For all samples, peptide and protein FDR were <1.1 and 0%, respectively.
2.11. Bioinformatic Analyses of Protein Subcellular Localization
Bioinformatic approaches were applied to proteins identified from the F. tularensis LVS genome sequence (accession number: NC_007880). Subcellular protein localizations were predicted with PSORTb (ver. 3.0.2) (http://www.psort.org/psortb). Classical signal peptides were detected with SignalP (ver. 4.1) (http://www.cbs.dtu.dk/services/SignalP), and signal peptides of lipoproteins were predicted with LipoP (ver. 1.0) (http://www.cbs.dtu.dk/services/LipoP). Nonclassically secreted proteins were predicted with SecretomeP (ver. 2.0) (http://www.cbs.dtu.dk/services/SecretomeP).
3. Results and Discussion
3.1. Characterization of the MPF Proteome
To first determine the complement of proteins in the protective MPF component of F. tularensis, we performed proteome characterization via multidimensional LC–MS/MS on a tryptic digest of MPF. This resulted in the identification of 284 proteins with a high degree of confidence (Table S1 in the Supporting Information). Bioinformatic analyses indicated that 40% (114 out of 284) of these proteins contained at least one signature or motif consistent with membrane or surface localization (translocated, 67 proteins; cell envelope localization, 58 proteins; or β-barrel motif, seven proteins). The remaining proteins were predicted to have a cytosolic or an unknown subcellular localization. The identification of a large number of predicted nonmembrane or nontranslocated proteins was not unexpected because experimental detection of presumably cytosolic proteins in bacterial membrane preparations is commonly reported.19,20 Of the 170 proteins not predicted to be membrane associated, 41 were described in previous studies as localized to the cell envelope (membrane, secreted, or surface protein). (See Table S1 in the Supporting Information.) In addition to adding validity to the identification and localization of the proteins comprising the MPF, the bioinformatics analyses served as a filter to reduce the complexity of the potential immunogens in MPF by 55% (155 of 284 proteins with predicted or experimentally determined membrane localization).
3.2. Sera from MPF Immunization and F. tularensis LVS Vaccination Recognize a Small Subset of MPF Proteins
We previously established that passive transfer of sera from mice immunized with MPF protects against a F. tularensis SCHU S4 challenge in a murine model and shows reactivity to specific components of MPF by 1D-Western blot analyses.7 To fully identify the immunoreactive proteins of MPF and further narrow the number of potential immunogen candidates, 2D-Western blots were probed with immune sera. 2D-PAGE analyses of MPF resulted in the resolution of 295 and 271 individual protein spots using pH 4–7 and pH 6–11 IEF gradients, respectively (Figure S1A and S1B in the Supporting Information). This was in concordance with a previous report, indicating a similar number of spots in F. tularensis membrane preparations.21 Proteins with close-to-neutral isoelectric points resolved effectively with the pH 4–7 IEF gradient (Figure S1A in the Supporting Information); however, only proteins with higher isoelectric points were well-resolved with a basic pH gradient (Figure S1B in the Supporting Information).
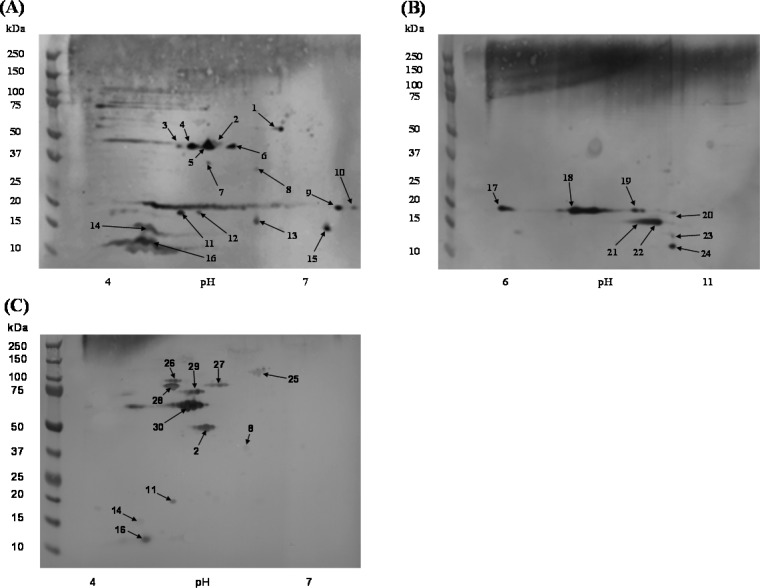
Western blot analyses with MPF immune serum revealed 16 and 8 immunoreactive protein spots in MPF preparations resolved with the pH 4–7 and pH 6–11 IEF gradients, respectively (Figure 2A,B). Additionally, immune serum from mice vaccinated with F. tularensis LVS was evaluated to compare effective humoral-based immune responses targeting MPF components.22 Immune serum from mice vaccinated with F. tularensis LVS was reactive to 11 protein spots (Figure 2C), of which five were also recognized by the anti-MPF immune sera.
Figure 2.
2D Western blot of MPF and F. tularensis LVS immune sera to MPF proteins. (A) MPF resolved in a pH range of 4–7 and probed with MPF immune sera. (B) MPF resolved in a pH range of 6–11 and probed with MPF immune sera. (C) MPF resolved in a pH range of 4–7 and probed with F. tularensis LVS immune sera. The numbered arrows correspond to the spots labeled in Figure 1 and the protein identifications presented in Table 1 and Table S2 in the Supporting Information.
Thirty immuno-reactive protein spots (those numbered in Figure S1 in the Supporting Information and Figure 2) were subject to LC–MS/MS analyses for protein identification (Table 1 and Table S2 in the Supporting Information). Q-TOF-based identifications were successful for 18 of these protein spots, and six additional spots (spots 1, 13, 17, 20, 23 and 24) of low protein abundance were identified using an Orbitrap platform. In total, 45 proteins were identified from the immunoproteome analyses, thus significantly reducing the number of potential target immunogens. Multiple proteins were identified in 10 of the protein spots (spots 1, 6, 13, 17, 20, 22–24, 29, and 30). In particular, protein spots 17, 20, and 23 were composed of a large number of proteins (7, 14, and 5 proteins, respectively), but this was not unexpected given the poor resolution in these areas of the 2D-gels (Figure S1 in the Supporting Information). A majority of the proteins identified from 2D-gels were also identified in the multidimensional LC–MS/MS analyses of MPF (30 proteins). Fifteen proteins detected from 2D-gels but not identified in the multidimensional LC–MS/MS approach were present in protein spots 1, 17, 20, 23, 24, and 26. Thus, the total number of MPF proteins was increased to 299 (Figure 1).
Table 1. Proteins Identified in MPF Spots.
| spot no. | protein name | locusa | signal peptideb | PSORTb localization (score)c | sera reactivityd | previously identified as immunoreactive and reference | previously identified as membrane (M), secreted (T), or surface (S) protein and reference |
|---|---|---|---|---|---|---|---|
| 1 | dihydrolipoamide dehydrogenase (Lpd)e | FTL_0311 | Cyto (9.97) | X | (21) | M21,37 | |
| ATP-dependent protease, ATP-binding subunit (HslU) | FTL_0964 | Cyto (9.97) | |||||
| glyceraldehyde-3-phosphate dehydrogenase (GapA)f,e | FTL_1146 | Cyto (9.97) | (38−40) | T35,41 | |||
| glutathione reductase (Gor)e | FTL_1248 | SpI | unknown | ||||
| 2 | elongation factor Tu (TufA)f | FTL_1751 | Cyto (9.97) | X, Y | (21,38,40,42−46) | M21 | |
| 3–4 | could not be identified with confidence | X | |||||
| 5 | outer membrane associated protein FopA1f | FTL_1328 | SpI | OM (9.93) | X | (21,38,40,42,45,46) | M,21,23,47,48 T,35 S25,28 |
| 6 | universal stress protein (Usp) | FTL_0166 | Cyto (9.97) | X | (39) | M37 | |
| outer membrane associated protein FopA1f | FTL_1328 | SpI | OM (9.93) | (21,38,40,42,45,46) | M,21,23,47,48 T,35 S25,28 | ||
| 7 | could not be identified with confidence | X | |||||
| 8 | outer membrane associated protein FopA1f | FTL_1328 | SpI | OM (9.93) | X, Y | (21,38,40,42,45,46) | M,21,23,47,48 T,35 S25,28 |
| 9–10 | could not be identified with confidence | X | |||||
| 11 | acetyl-CoA carboxylase, biotin carboxyl carrier protein subunit (AccB)f | FTL_1592 | unknown | X, Y | (21,39,40,43−46) | M21,47 | |
| 12 | acetyl-CoA carboxylase, biotin carboxyl carrier protein subunit (AccB)f | FTL_1592 | unknown | X | (39,40,43−46) | M21,47 | |
| 13 | hypothetical proteinf | FTL_0617 | Cyto (8.96) | X | (21,38,39,45) | M,21,37,49 T,35,41 S25 | |
| 50S ribosomal protein L9 (RplI)f | FTL_1026 | Cyto (9.97) | (38,40,45,46) | T35 | |||
| F0F1 ATP synthase subunit delta (AtpH) | FTL_1798 | Cyto (9.26) | M49 | ||||
| 14 | 50S ribosomal protein L7/L12 (RplL)f | FTL_1745 | unknown | X, Y | (21,38−40,42,46) | M21 | |
| 15 | F0F1 ATP synthase subunit epsilon (AtpC) | FTL_1794 | NC (SP) | unknown | X | M49 | |
| 16 | hypothetical protein | FTL_0105 | SpI | unknown | X, Y | (40,45,46) | M48 |
| 17 | 30S ribosomal protein S7 (RpsG) | FTL_0233 | Cyto (9.97) | X | |||
| 50S ribosomal protein L5 (RplE) | FTL_0248 | Cyto (9.97) | |||||
| 30S ribosomal protein S5 (RpsE)e | FTL_0253 | NC (SP) | Cyto (9.26) | (46) | |||
| 50S ribosomal protein L15 (RplO) | FTL_0255 | NC (SP) | Cyto (9.26) | ||||
| outer membrane protein OmpHf,e | FTL_0536 | SpI | unknown | (38,40) | M37,47 | ||
| (3R)-hydroxymyristoyl-ACP dehydratase (FabZ)e | FTL_0538 | Cyto (9.97) | |||||
| 50S ribosomal protein L13 (RplM) | FTL_1187 | NC (SP) | Cyto (9.26) | ||||
| 18 | hypothetical protein | FTL_0571 | SpII | unknown | X | (45) | M47 |
| 19 | could not be identified with confidence | X | |||||
| 20 | 30S ribosomal protein S7 (RpsG) | FTL_0233 | Cyto (9.97) | X | |||
| 50S ribosomal protein L16 (RplP) | FTL_0243 | Cyto (9.97) | |||||
| 50S ribosomal protein L5 (RplE) | FTL_0248 | Cyto (9.26) | |||||
| 30S ribosomal protein S8 (RpsH) | FTL_0250 | Cyto (9.26) | |||||
| 30S ribosomal protein S5 (RpsE)e | FTL_0253 | NC (SP) | Cyto (9.26) | (46) | |||
| 50S ribosomal protein L15 (RplO) | FTL_0255 | NC (SP) | Cyto (9.26) | ||||
| 50S ribosomal protein L17 (RplQ) | FTL_0262 | Cyto (9.97) | |||||
| hypothetical protein (annotated as a pseudogene)e | FTL_0349 | unknown | |||||
| peptide methionine sulfoxide reductase (MsrB)e | FTL_0379 | SpI | Cyto (9.26) | ||||
| lipoprotein (LpnA)f | FTL_0421 | SpII | OM (10.00) | (21,40,43,44,46) | M,21,23,47,49 S26 | ||
| 30S ribosomal protein S9 (RpsI) | FTL_1186 | NC (SP) | Cyto (9.97) | ||||
| 50S ribosomal protein L10 (RplJ) | FTL_1746 | Cyto (9.26) | M37 | ||||
| 50S ribosomal protein L11 (RplK)e | FTL_1748 | NC (SP) | Cyto (9.26) | T35 | |||
| Sau5/YciO/YrdC family proteine | FTL_1913 | unknown | |||||
| 21 | lipoprotein (LpnA)f | FTL_0421 | SpII | OM (10.00) | X | (21,40,43,44,46) | M,21,23,47,49 S26 |
| 22 | lipoprotein (LpnA)f | FTL_0421 | SpII | OM (10.00) | X | (21,40,43,44,46) | M,21,23,47,49 S26 |
| 50S ribosomal protein L10 (RplJ) | FTL_1746 | Cyto (9.26) | M37 | ||||
| 23 | 50S ribosomal protein L10 (RplJ) | FTL_0235 | Cyto (9.26) | X | |||
| 50S ribosomal protein L22 (RplV)e | FTL_0241 | Cyto (9.26) | |||||
| 50S ribosomal protein L24 (RplX) | FTL_0247 | Cyto (9.97) | |||||
| hypothetical proteinf | FTL_0617 | Cyto (8.96) | (21,38,39,45) | M,21,37,49 T,35,41 S25 | |||
| histone-like protein HU form B (HupB)e | FTL_0895 | NC (SP) | Cyto (9.26) | (21) | M,21 T35 | ||
| 24 | histone-like protein HU form B (HupB)e | FTL_0895 | NC (SP) | Cyto (9.26) | X | (21) | M,21 T35 |
| 50S ribosomal protein L31 (RpmE)e | FTL_1303 | NC (SP) | Cyto (9.26) | ||||
| 30S ribosomal protein S16 (RpsP)e | FTL_1738 | Cyto (9.26) | |||||
| 25 | pyruvate dehydrogenase, E1 component (AceE) | FTL_0309 | Cyto (9.97) | Y | (40,50) | M23,50 | |
| 26 | chitinase family 18 protein (ChiA)f,e | FTL_1521 | SpI | unknown | Y | (38,45,46) | M23 |
| 27 | peroxidase/catalase (KatG)f | FTL_1504 | NC (SP) | Cyto (9.26) | Y | (21,38−40,42,45,46,50) | M,21,23,47,50 T35,41 |
| 28 | chaperone protein DnaKf | FTL_1191 | NC (SP) | Cyto (9.97) | Y | (21,38−40,42−46,50) | M,21,23,50 T,35,41 S25 |
| 29 | dihydrolipoamide acetyltransferase (AceF) | FTL_0310 | NC (SP) | Cyto (9.97) | Y | (21,40,45,50) | M21,37,47,50 |
| outer membrane associated protein FopA1f | FTL_1328 | SpI | OM (9.93) | (21,38,40,42,45,46) | M,21,23,47,48 T,35 S25,28 | ||
| chaperonin GroELf | FTL_1714 | Cyto (9.97) | (21,38−40,42−46) | M,21,23,43 T,35,41 S25 | |||
| 30 | chaperonin GroELf | FTL_1714 | Cyto (9.97) | Y | (21,38−40,42−46) | M,21,23,43 T,35,41 S25 | |
| dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase complex (SucB) | FTL_1783 | Cyto (9.97) | (21,40,45,46,50) | M21,37,50 |
NCBI reference sequence identification codes matching each loci are listed in Table S2 in the Supporting Information.
Signal peptide prediction; SpI, signal peptide cleaved by signal peptidase I; SpII, signal peptide cleaved by signal peptidase II; NC (SP), nonclassical signal peptide.
Subcellular localization predicted with PSORTb. Peri, periplasmic; Cyto, cytosol; OM, outer membrane; CM, cytoplasmic membrane.
X and Y designate proteins were reactive to sera from mice immunized with MPF and F. tularensis LVS vaccination, respectively.
Protein was not identified in the multidimensional LC–MS/MS analysis of MPF.
Protein was identified on the surface of F. tularensis LVS in this study.
The dominant antigens reactive to the MPF immune sera displayed a mass of <50 kDa. Single proteins were identified in protein spots 2, 5, 8, 11, 12, 14–16, 18, 21, and 25–28. Protein spot 5 was identified as outer-membrane-associated protein (FopA1, FTL_1328). Spot 8 (∼36 kDa) was also identified as FopA1, despite its lower observed molecular mass as compared with spot 5 (∼43 kDa). This finding was in agreement with a previous report where immunoreactive FopA1 resolved at multiple molecular masses and similar isoelectric points.23 When multiple proteins were identified in a single spot (spots 1, 6, 13, 17, 20, 22–24, 29, and 30), the identification of the specific anti-MPF reactive antigen(s) was difficult. Nonetheless, previously identified immunoreactive proteins were present in each of these spots. (See Table 1.) The proteins of six immunoreactive spots (spots 3, 4, 7, 9, 10, and 19) could not be identified with confidence, presumably due to low abundance (Figure S1A in the Supporting Information and Figure 1B). Several areas (∼20 kDa and 75 to 100 kDa) of the anti-MPF pH 4–7 MPF Western blot displayed immunoreactivities defined by a smear (Figure 2A). These smears occurred in areas with minimal protein, as detected by silver staining (Figure S1A in the Supporting Information), and thus were not pursued for protein identification.
When F. tularensis LVS immune sera was used to probe 2D-Western blots of MPF, there were fewer immunoreactive protein spots as compared with the MPF immune sera (compare Figure 2A,B to 2C). These 11 protein spots (spots 2, 8, 11, 14, 16, and 25–30) resolved only in the pH range of 4–7 and were predominantly of a mass greater than or equal to 50 kDa (spots 2 and 25–30) (Figure 2C). From these spots, 15 proteins were identified by MS (Table 1). The proteins in spots 25–30 were uniquely recognized by the F. tularensis LVS immune sera. Five protein spots were reactive with both MPF and F. tularensis LVS immune sera (spots 2, 8, 11, 14, and 16).
3.3. Surface-Exposed Proteome of F. tularensis LVS
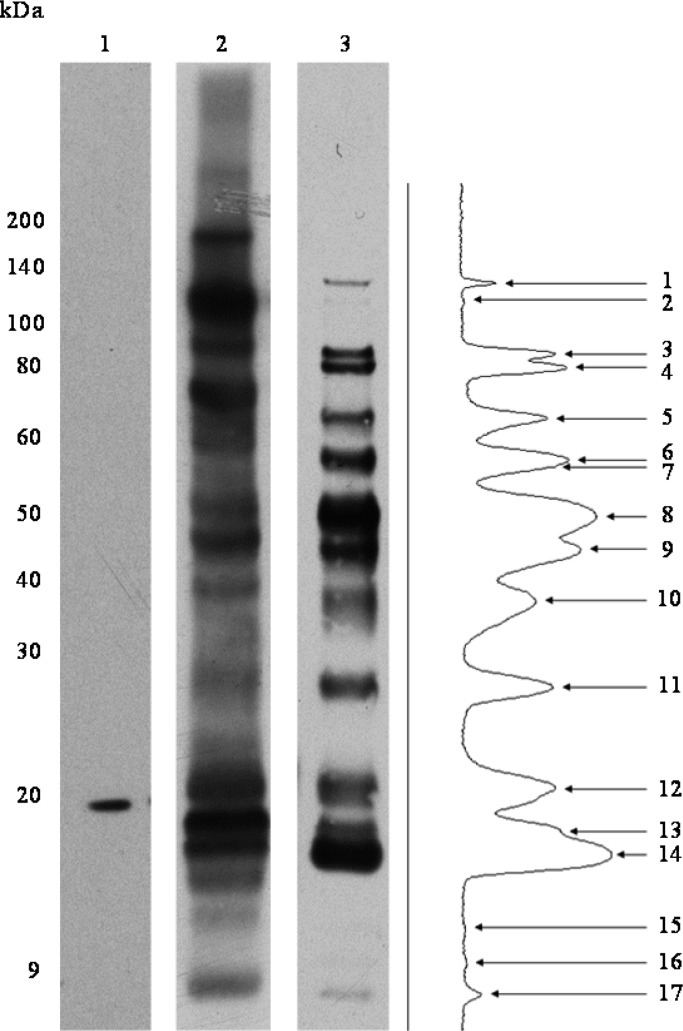
Immunogenic proteins are often exposed on the surface of bacteria, and we hypothesized that this is true for the proteins targeted by the protective IgG response against F. tularensis.(12,13) Bioinformatics analyses of the 45 MPF proteins identified in immunoproteome analyses predicted that 71.1% (32 of 45) could be surface-localized. This was an overall 89% reduction in the potential MPF target immunogens (Figure 1). However, the bioinformatics applied were not specifically designed to predict surface localization. As such, a direct analysis of surface protein localization on F. tularensis LVS was performed using a membrane-impermeable biotin label, LC-Biotin. Initial experiments to optimize surface labeling were performed with LC-Biotin concentrations in a range between 6.6 and 26.4 mg/mL, with incubation times of 10, 30, 60, and 120 min (data not shown). This established that treatment of freshly harvested F. tularensis with 6.6 mg/mL LC-Biotin for 60 min provided optimal labeling. After surface labeling of F. tularensis LVS, the proteins were extracted from the cells and detected by Western blot using an HRP-conjugated antibiotin antibody as the probe (Figure 3, lane 3). This resulted in the detection of 17 distinct bands. In comparison, labeling of F. tularensis LVS cytosolic proteins as a control resulted in a different and more complex pattern of proteins (Figure 3, lane 2), thus providing further evidence that labeling of intact cells was selective for surface-exposed products.
Figure 3.
Biotinylation of F. tularensis LVS surface-exposed proteins. Antibiotin Western blots to detect biotinylated proteins after labeling F. tularensis LVS surface proteins with LC-Biotin. Lane 1, WCL of F. tularensis LVS unlabeled control; Lane 2, labeled F. tularensis LVS WCL (15 s exposure); Lane 3, labeled F. tularensis LVS intact cells (2 min exposure) accompanied by densitometry analysis of reactive protein bands.
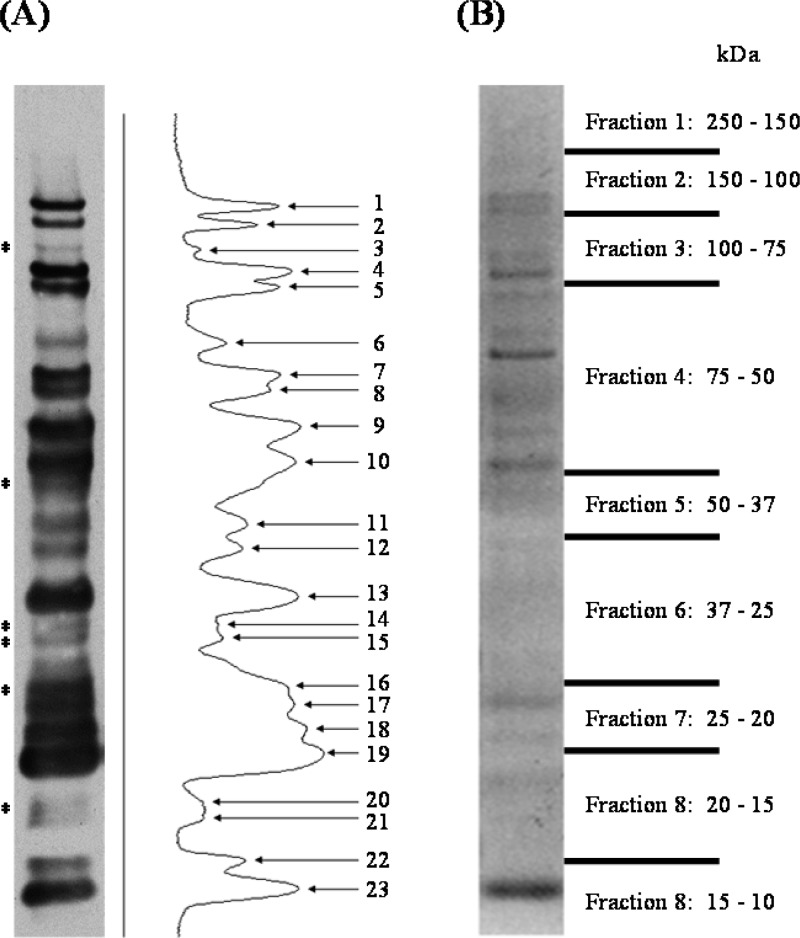
Identification of the surface proteins was facilitated by purification of the biotin-tagged products with immobilized streptavidin affinity chromatography. Antibiotin Western blot analysis of the purified material demonstrated the enrichment of 23 antibiotin reactive bands (Figure 4A). An aliquot of affinity-purified material was resolved by SDS-PAGE, the gel was cut into nine slices based on molecular mass (Figure 4B), and LC–MS/MS-based protein identification resulted in 31 proteins (Table 2 and Table S3 in the Supporting Information). A positive correlation was observed between the calculated molecular mass of each protein identified and the observed molecular mass based on SDS-PAGE migration. Of the 31 identified surface-exposed proteins, 27 were detected in the MPF by multidimensional LC–MS/MS and 2D-PAGE. A bioinformatic analysis including previous literature and predictive algorithms revealed only 4 of the 31 identified surface proteins lacked supporting evidence of membrane/surface localization (Table 2). Prior studies directly identified 12 F. tularensis or F. novicida proteins as surface-localized.24−29 However, only five of these were identified by our surface-labeling analyses (Table 2) and also were the only previously defined surface proteins identified in the immunoproteome analyses (Table 1).
Figure 4.
Purified F. tularensis LVS LC-Biotin labeled surface proteins. Biotinylated surface proteins purified using immobilized streptavidin were separated by SDS-PAGE and analyzed by antibiotin Western blot. (A) Antibiotin Western blot of purified biotinylated surface proteins accompanied by densitometry analysis of reactive protein bands. (B) Simply Blue stained gel of affinity-purified material. Gel fractions excised for LC–MS/MS analysis are denoted on the right by the molecular mass range. *indicates reactive protein bands only visualized after affinity purification.
Table 2. Surface-Associated Proteins of F. tularensis LVS.
| protein name | locusa | signal peptideb | PSORTb localization (score)c | sera reactivityd | previously identified as membrane (M), secreted (T), or surface (S) protein and reference |
|---|---|---|---|---|---|
| Group 1 Surface Immunogense | |||||
| outer membrane associated protein FopA1f | FTL_1328 | SpI | OM (9.93) | X,Y | M,21,23,47,48 T,35 S25,28 |
| acetyl-CoA carboxylase, biotin carboxyl carrier protein subunit (AccB)f | FTL_1592 | unknown | X,Y | M21,47 | |
| 50S ribosomal protein L7/L12 (RplL)f | FTL_1745 | unknown | X,Y | M21 | |
| elongation factor Tu (TufA)f | FTL_1751 | Cyto (9.97) | X,Y | M21 | |
| Group 2 and 3 Surface Immuogense | |||||
| lipoprotein (LpnA)f | FTL_0421 | SpII | OM (10.00) | X | M,21,23,47,49 S26 |
| outer membrane protein OmpHf | FTL_0536 | SpI | unknown | X | M37,47 |
| hypothetical proteinf | FTL_0617 | Cyto (8.96) | X | M,21,37,49 T,35,41 S25 | |
| 50S ribosomal protein L9 (RplI)f | FTL_1026 | Cyto (9.97) | X | T35 | |
| glyceraldehyde-3-phosphate dehydrogenase (GapA)f | FTL_1146 | Cyto (9.97) | X | T35,41 | |
| chaperone protein DnaKf | FTL_1191 | NC (SP) | Cyto (9.97) | Y | M,21,23,50 T,35,41 S25 |
| peroxidase/catalase (KatG)f | FTL_1504 | NC (SP) | Cyto (9.26) | Y | M,21,23,47,50 T35,41 |
| chitinase family 18 protein (ChiA)f | FTL_1521 | SpI | unknown | Y | M23 |
| chaperonin GroELf | FTL_1714 | Cyto (9.97) | Y | M,21,23,43 T,35,41 S25 | |
| Nonimmunoreactive Surface Proteins | |||||
| outer membrane protein | FTL_0009 | SpI | Peri (9.84) | M,21,37,47,49 T35 | |
| intracellular growth locus, subunit B (IglB)f | FTL_0112 | Cyto (9.97) | M50 | ||
| intracellular growth locus, subunit C (IglC)f | FTL_0113 | unknown | M,21,37 T51 | ||
| elongation factor G (FusA)f | FTL_0234 | Cyto (10.00) | |||
| heat shock protein 90 (HtpG)f | FTL_0267 | Cyto (9.97) | |||
| glutamate dehydrogenase (Gdh)f | FTL_0269 | unknown | M,37 T35 | ||
| OmpA family proteinf | FTL_0325 | SpII | unknown | M21,23,47 | |
| peptidoglycan-associated lipoproteinf | FTL_0336 | SpII | OM (10.00) | M23,47−49 | |
| hypothetical proteinf | FTL_0569 | SpI | unknown | ||
| AhpC/TSA family proteinf | FTL_1015 | NC (SP) | unknown | M,21 T35,41 | |
| lipoprotein (DsbG, FipB)f | FTL_1096 | SpII | unknown | M23,37,47−49,52 | |
| hypothetical protein | FTL_1225 | SpII | unknown | ||
| hypothetical protein | FTL_1494 | SpII | unknown | ||
| succinyl-CoA synthetase subunit beta (SucC)f | FTL_1553 | Cyto (8.96) | M,50 T41 | ||
| aconitate hydratase (AcnA)f | FTL_1772 | Cyto (9.97) | M21,37,50 | ||
| citrate synthase (GltA) | FTL_1789 | Cyto (9.97) | |||
| cell division protein FtsZf | FTL_1907 | Cyto (9.12) | M21,49 | ||
| 30S ribosomal protein S1 (RpsA)f | FTL_1912 | Cyto (9.97) | |||
NCBI reference sequence identification codes matching each loci are listed in Table S3 in the Supporting Information.
Signal peptide prediction; SpI, signal peptide cleaved by signal peptidase I; SpII, signal peptide cleaved by signal peptidase II; NC (SP), nonclassical signal peptide.
Subcellular localization predicted with PSORTb; Peri, periplasmic; Cyto, cytosol; OM, outer membrane; CM, cytoplasmic membrane.
X and Y designate proteins were reactive to sera from mice immunized with MPF and F. tularensis LVS vaccination, respectively.
Group 1 immunogens are jointly recognized by F. tularensis LVS immune sera, group 2 immunogens by F. tularensis LVS immune sera, and group 3 immunogens by MPF immune sera.
Indicates the protein was identified in the MPF by multidimensional LC–MS/MS or from MPF 2D-PAGE protein spots.
The results of the predictive bioinformatic algorithms as well as predicted function indicate that nearly half of the 31 identified surface proteins could be classified as cytosolic products. Thus, the application of a reverse vaccinology immunogen selection approach based solely on bioinformatics algorithms for subcellular localization would have resulted in greater ambiguity in target immunogen selection. As with membrane proteins, experimental identification of known or predicted cytosolic proteins in subcellular fractions of surface proteins is not uncommon. There is a growing list of presumed intracellular proteins identified on the surface of pathogenic bacteria that appear to be multifunctional based on their subcellular location.30 For example, we identified glycolytic enzyme 2-phosphoglycerate dehydratase GapA (FTL_1146) as being surface-exposed. This is consistent with findings in both Gram-negative and Gram-positive pathogens where this protein was demonstrated to participate in bacterial adhesion and was identified on cell surfaces despite lacking predicted signal peptides.19,31 Likewise, TufA (FTL_1751) was observed as surface-associated, and homologues of this gene product in Lactobacillus, Mycoplasma, and Pseudomonas species are described as acting as adhesions and plasminogen ligands on the bacterial surface.20,32 The bacterial GroEL and DnaK chaperones or stress response proteins are also known to interact with the innate immune response and enhance antigen presentation.33 Although they are cytosolic functioning chaperones, they have been noted as surface-exposed in other bacteria.34 Thus, the surface presentation of GroEL (FTL_1714) and chaperone protein DnaK (FTL_1191) in F. tularensis was not unexpected. Recently, Konecna et al. identified 22 predicted cytosolic proteins that were expelled into the culture supernatant by F. tularensis LVS and SCHU S4;35 four of these were found in our analyses as surface products of F. tularensis LVS including GroEL (FTL_1714), glutamate dehydrogenase (Gdh, FTL_0269), GapA (FTL_1146), and hypothetical protein FTL_0617. An alternative explanation for identification of predicted cytosolic proteins as surface structures is unexpected membrane permeability of the LC-Biotin reagent and labeling of the most abundant cytosolic proteins. However, this seems unlikely as the protein profile obtained from cell surface labeling was markedly different from that obtained by LC-Biotin labeling of WCL. It is also possible that some cytosolic contaminants may have been copurified with biotinylated surface proteins.
3.4. Grouping and Prioritization of MPF Immunogens
When F. tularensis LVS surface protein characterization was combined with the bioinformatics and the immunoproteome analyses, the potential number of immunotherapeutic targets in MPF is reduced from a total of 299 to 13 (Figure 1 and Table 2), a 96% reduction in target complexity. However, the further grouping of MPF surface immunogens based on reactivity to MPF and F. tularensis LVS immune sera provides an additional means to prioritize the testing of multiple purified proteins in a postexposure vaccine model. Such immunogen grouping also enables the ability to test whether postexposure vaccine candidates can be identified and selected based on differential reactivity to various immunization approaches.
The immunoreactive proteins of the MPF could be placed into three seroreactive groups: group-1, proteins recognized by both MPF and F. tularensis LVS immune sera; group-2, proteins recognized specifically by F. tularensis LVS immune sera; and group-3, proteins recognized specifically by MPF immune sera.
Five proteins of MPF fell into group-1: TufA (FTL_1751); FopA1 (FTL_1328); acetyl-CoA carboxylase, biotin carboxyl carrier protein subunit (AccB, FTL_1592); 50S ribosomal protein L7/L12 RplL (FTL_1745); and hypothetical protein FTL_0105 (Table 1). All but one of these proteins (FTL_0105) were also identified as surface antigens of F. tularensis LVS (Table 2). Of the group-1 MPF products, FopA1 has been the most extensively evaluated as a surface-exposed immunogen of F. tularensis.9,23,25 FopA1 and anti-FopA1 antibodies provided prophylactic protection against a lethal F. tularensis LVS challenge in mice but not against type A F. tularensis.10,36 Huntley et al. demonstrated that immunization with an outer membrane protein adjuvant complex, including FopA1, produced high titers of IgM and IgG (IgG2a and IgG3) and provided a significant level of protection against type A F. tularensis.(9) This suggested that a multiple immunogen complex including FopA1 is required to elicit prophylactic protection. We hypothesize the same would be required for a immunotherapeutic vaccine, and the group-1 proteins represent those selected by the reverse vaccinology approach as being the highest priority to test.
Eight proteins were identified in the six immunoreactive spots that were exclusively reactive to sera from vaccination with F. tularensis LVS (Table 1). Of these eight group-2 proteins, four were also identified as surface-localized (Table 2): DnaK (FTL_1191), KatG (FTL_1504), ChiA (FTL_1521), and GroEL (FTL_1714). Given the demonstrated effectiveness of F. tularensis LVS vaccination, it could be hypothesized that the F. tularensis LVS immunoreactive proteins might also be effective as immunotherapeutic targets. Additionally, it is noted that all group-2 proteins were previously recognized as immunogens (Table 1).
A total of 35 proteins were identified in the 13 protein spots immunoreactive only to the anti-MPF sera (group-3), with 12 of these previously identified as antigens (Table 1). The inclusion of surface-labeling data reduced the number of potential group-3 immunotherapeutic targets to five: LpnA (FTL_0421), hypothetical protein FTL_0617, RplI (FTL_1026), OmpH (FTL_0536), and GapA (FTL_1146). The most notable of these is LpnA (FTL_0421), which has previously been studied as a subunit vaccine immunogen.10
4. Conclusions
Our previous studies demonstrated that passive transfer of sera from MPF immunized mice to naïve F. tularensis SCHU S4 infected animals enhanced gentamicin therapy and the sera possessed high titers of IgM and IgG (IgG2a and IgG3), targeting LPS and MPF proteins, respectively.7 Moreover, it was observed that with the MPF postexposure vaccination the presence of an IgG response at day-7 corresponded to initial recovery from infection.7 This work confirmed that the humoral immune response significantly contributes to the control and clearance of F. tularensis infections.5,9,10 However, it did not define the molecular identity of proteins that were immunogenic with MPF postexposure vaccination. In this present study, we characterized the MPF proteome as being composed of at least 299 proteins, identified the repertoire of MPF proteins recognized by MPF immunization, and compared these with the MPF proteins recognized by F. tularensis LVS vaccination. This reduced the number of target immunogens to 45, and bioinformatics indicated that 32 of these had the potential to be surface-localized. To further reduce the complexity of immunogens prioritized as immunotherapeutic targets, a direct analysis of F. tularensis LVS surface proteins was performed. This reduced the complexity of target immunogens to 13. Thus, comparative proteomics evaluations of MPF based on serological reactivity and surface localization provided a means to select and prioritize potential immunogens for further evaluation. From the collective data, we hypothesize that 13 proteins on the surface of F. tularensis and found to be immunoreactive compose the primary candidates for a defined postexposure vaccine. These 13 candidates are FopA1 (FTL_1328), AccB (FTL_1592), RplL (FTL_1745), TufA (FTL_1751), OmpH (FTL_0536), LpnA (FTL_0421), FTL_0617, RplI (FTL_1026), GapA (FTL_1146), DnaK (FTL_1191), KatG (FTL_1504), ChiA (FTL_1521), and GroEL (FTL_1714).
Acknowledgments
This research was supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases grant: U54 AI-065357.
Glossary
Abbreviations
- MPF
membrane protein fraction
- type A
F. tularensis subsp. tularensis
- type B
F. tularensis subsp. holarctica (type B)
- LVS
live vaccine strain
- CLDC
cationic liposomal DNA complex
- LC-biotin
EZ-Link sulfo-NHS-LC-biotin
- WCL
whole cell lysate
Supporting Information Available
Table S1: Complete list of the 284 proteins identified in MPF by multidimensional LC–MS/MS, accompanied by corresponding MS/MS data and bioinformatic predictions. Table S2: LC–MS/MS analyses of MPF 2D-PAGE resolved protein spots. Table S3: LC–MS/MS analyses of the identified surface proteins. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
⊥ J.C.C.: Department of Animal Science, University of Wyoming, 1000 East University Avenue, Laramie, WY 82071.
Author Contributions
∥ J.C.C. and M.D.S. contributed equally.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Saslaw S.; Eigelsbach H. T.; Wilson H. E.; Prior J. A.; Carhart S. Tularemia vaccine study. I. Intracutaneous challenge. Arch. Intern. Med. 1961, 107, 689–701. [DOI] [PubMed] [Google Scholar]
- Saslaw S.; Eigelsbach H. T.; Prior J. A.; Wilson H. E.; Carhart S. Tularemia vaccine study. II. Respiratory challenge. Arch. Intern. Med. 1961, 107, 702–714. [DOI] [PubMed] [Google Scholar]
- Dennis D. T.; Inglesby T. V.; Henderson D. A.; Bartlett J. G.; Ascher M. S.; Eitzen E.; Fine A. D.; Friedlander A. M.; Hauer J.; Layton M.; Lillibridge S. R.; McDade J. E.; Osterholm M. T.; O’Toole T.; Parker G.; Perl T. M.; Russell P. K.; Tonat K. Tularemia as a biological weapon: medical and public health management. JAMA, J. Am. Med. Assoc. 2001, 285212763–2773. [DOI] [PubMed] [Google Scholar]
- Foshay L.; Hesselbrock W. H.; Wittenberg H. J.; Rodenberg A. H. Vaccine prophylaxis against tularemia in man. Am. J. Public Health Nations Health 1942, 32101131–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabick J. J.; Narayanan R. B.; Williams J. C.; Leduc J. W.; Nacy C. A. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am. J. Med. Sci. 1994, 308283–87. [DOI] [PubMed] [Google Scholar]
- Mara-Koosham G.; Hutt J. A.; Lyons C. R.; Wu T. H. Antibodies contribute to effective vaccination against respiratory infection by type A Francisella tularensis strains. Infect. Immun. 2011, 7941770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland M. D.; Goodyear A. W.; Troyer R. M.; Chandler J. C.; Dow S. W.; Belisle J. T. Post-exposure immunization against Francisella tularensis membrane proteins augments protective efficacy of gentamicin in a mouse model of pneumonic tularemia. Vaccine 2012, 30334977–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland R.; Olivares-Zavaleta N.; Warawa J. M.; Gherardini F. C.; Jarrett C.; Hinnebusch B. J.; Belisle J. T.; Fairman J.; Bosio C. M. Effective, broad spectrum control of virulent bacterial infections using cationic DNA liposome complexes combined with bacterial antigens. PLoS Pathog. 2010, 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley J. F.; Conley P. G.; Rasko D. A.; Hagman K. E.; Apicella M. A.; Norgard M. V. Native outer membrane proteins protect mice against pulmonary challenge with virulent type A Francisella tularensis. Infect. Immun. 2008, 7683664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitt A. G.; Mena-Taboada P.; Monsalve G.; Benach J. L. Francisella tularensis infection-derived monoclonal antibodies provide detection, protection, and therapy. Clin. Vaccine Immunol. 2009, 163414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J. W.; Shen H.; Webb A.; Perry M. B. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine 2002, 2029–303465–3471. [DOI] [PubMed] [Google Scholar]
- Donati C.; Rappuoli R. Reverse vaccinology in the 21st century: improvements over the original design. Ann. N.Y. Acad. Sci. 2013, 1285, 115–32. [DOI] [PubMed] [Google Scholar]
- Pizza M.; Scarlato V.; Masignani V.; Giuliani M. M.; Arico B.; Comanducci M.; Jennings G. T.; Baldi L.; Bartolini E.; Capecchi B.; Galeotti C. L.; Luzzi E.; Manetti R.; Marchetti E.; Mora M.; Nuti S.; Ratti G.; Santini L.; Savino S.; Scarselli M.; Storni E.; Zuo P.; Broeker M.; Hundt E.; Knapp B.; Blair E.; Mason T.; Tettelin H.; Hood D. W.; Jeffries A. C.; Saunders N. J.; Granoff D. M.; Venter J. C.; Moxon E. R.; Grandi G.; Rappuoli R. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 2000, 28754591816–1820. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 2275259680–685. [DOI] [PubMed] [Google Scholar]
- Sonnenberg M. G.; Belisle J. T. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect. Immun. 1997, 65114515–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartain M. J.; Belisle J. T. N-Terminal clustering of the O-glycosylation sites in the Mycobacterium tuberculosis lipoprotein SodC. Glycobiology 2009, 19138–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A.; Nesvizhskii A. I.; Kolker E.; Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74205383–5392. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii A. I.; Keller A.; Kolker E.; Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75174646–4658. [DOI] [PubMed] [Google Scholar]
- Pancholi V.; Fischetti V. A. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J. Exp Med. 1992, 1762415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato D.; Bergonzelli G. E.; Pridmore R. D.; Marvin L.; Rouvet M.; Corthesy-Theulaz I. E. Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infect. Immun. 2004, 7242160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janovska S.; Pavkova I.; Hubalek M.; Lenco J.; Macela A.; Stulik J. Identification of immunoreactive antigens in membrane proteins enriched fraction from Francisella tularensis LVS. Immunol. Lett. 2007, 1082151–159. [DOI] [PubMed] [Google Scholar]
- Elkins K. L.; Cowley S. C.; Bosio C. M. Innate and adaptive immunity to Francisella. Ann. N.Y. Acad. Sci. 2007, 1105, 284–324. [DOI] [PubMed] [Google Scholar]
- Huntley J. F.; Conley P. G.; Hagman K. E.; Norgard M. V. Characterization of Francisella tularensis outer membrane proteins. J. Bacteriol. 2007, 1892561–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludu J. S.; de Bruin O. M.; Duplantis B. N.; Schmerk C. L.; Chou A. Y.; Elkins K. L.; Nano F. E. The Francisella pathogenicity island protein PdpD is required for full virulence and associates with homologues of the type VI secretion system. J. Bacteriol. 2008, 190134584–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo A.; Sledjeski D. D.; Lipski S.; Wooten R. M.; Basrur V.; Lafontaine E. R. Identification of a Francisella tularensis LVS outer membrane protein that confers adherence to A549 human lung cells. FEMS Microbiol Lett. 2006, 2631102–108. [DOI] [PubMed] [Google Scholar]
- Sandstrom G.; Tarnvik A.; Wolf-Watz H. Immunospecific T-lymphocyte stimulation by membrane proteins from Francisella tularensis. J. Clin Microbiol. 1987, 254641–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund A. L.; Kuoppa K.; Svensson K.; Salomonsson E.; Johansson A.; Bystrom M.; Oyston P. C. F.; Michell S. L.; Titball R. W.; Noppa L.; Frithz-Lindsten E.; Forsman M.; Forsberg A. Direct repeat-mediated deletion of a type IV pilin gene results in major virulence attenuation of Francisella tularensis. Mol. Microbiol. 2006, 5961818–1830. [DOI] [PubMed] [Google Scholar]
- Nano F. E. Identification of a heat-modifiable protein of Francisella-tularensis and molecular-cloning of the encoding gene. Microb. Pathog. 1988, 52109–119. [DOI] [PubMed] [Google Scholar]
- Mahawar M.; Atianand M. K.; Dotson R. J.; Mora V.; Rabadi S. M.; Metzger D. W.; Huntley J. F.; Harton J. A.; Malik M.; Bakshi C. S. Identification of a novel Francisella tularensis factor required for intramacrophage survival and subversion of innate immune response. J. Biol. Chem. 2012, 2873025216–25229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V.; Chhatwal G. S. Housekeeping enzymes as virulence factors for pathogens. Int. J. Med. Microbiol. 2003, 2936391–401. [DOI] [PubMed] [Google Scholar]
- Tunio S. A.; Oldfield N. J.; Ala’Aldeen D. A.; Wooldridge K. G.; Turner D. P. The role of glyceraldehyde 3-phosphate dehydrogenase (GapA-1) in Neisseria meningitidis adherence to human cells. BMC Microbiol. 2010, 10, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert A.; Losse J.; Gruszin C.; Huhn M.; Kaendler K.; Mikkat S.; Volke D.; Hoffmann R.; Jokiranta T. S.; Seeberger H.; Moellmann U.; Hellwage J.; Zipfel P. F. Immune evasion of the human pathogen Pseudomonas aeruginosa: elongation factor Tuf is a factor H and plasminogen binding protein. J. Immunol. 2007, 17952979–2988. [DOI] [PubMed] [Google Scholar]
- Stewart G. R.; Young D. B. Heat-shock proteins and the host-pathogen interaction during bacterial infection. Curr. Opin. Immunol. 2004, 164506–510. [DOI] [PubMed] [Google Scholar]
- Schaumburg J.; Diekmann O.; Hagendorff P.; Bergmann S.; Rohde M.; Hammerschmidt S.; Jansch L.; Wehland J.; Karst U. The cell wall subproteome of Listeria monocytogenes. Proteomics 2004, 4102991–3006. [DOI] [PubMed] [Google Scholar]
- Konecna K.; Hernychova L.; Reichelova M.; Lenco J.; Klimentova J.; Stulik J.; Macela A.; Alefantis T.; DelVecchio V. G. Comparative proteomic profiling of culture filtrate proteins of less and highly virulent Francisella tularensis strains. Proteomics 2010, 10244501–4511. [DOI] [PubMed] [Google Scholar]
- Fulop M.; Manchee R.; Titball R. Role of Lipopolysaccharide and a major outer-membrane protein from Francisella-tularensis in the induction of immunity against tularemia. Vaccine 1995, 13131220–1225. [DOI] [PubMed] [Google Scholar]
- Pavkova I.; Reichelova M.; Larsson P.; Hubalek M.; Vackova J.; Forsberg A.; Stulik J. Comparative proteome analysis of fractions enriched for membrane-associated proteins from Francisella tularensis subsp. tularensis and F. tularensis subsp. holarctica strains. J. Proteome Res. 2006, 5113125–3134. [DOI] [PubMed] [Google Scholar]
- Havlasova J.; Hernychova L.; Brychta M.; Hubalek M.; Lenco J.; Larsson P.; Lundqvist M.; Forsman M.; Krocova Z.; Stulik J.; Macela A. Proteomic analysis of anti-Francisella tularensis LVS antibody response in murine model of tularemia. Proteomics 2005, 582090–2103. [DOI] [PubMed] [Google Scholar]
- Pelletier N.; Raoult D.; La Scola B. Specific recognition of the major capsid protein of Acanthamoeba polyphaga mimivirus by sera of patients infected by Francisella tularensis. FEMS Microbiol. Lett. 2009, 2971117–123. [DOI] [PubMed] [Google Scholar]
- Twine S.; Shen H.; Harris G.; Chen W. X.; Sjostedt A.; Ryden P.; Conlan W. BALB/c mice, but not C57BL/6 mice immunized with a Delta clpB mutant of Francisella tularensis subspecies tularensis are protected against respiratory challenge with wild-type bacteria: Association of protection with post-vaccination and post-challenge immune responses. Vaccine 2012, 30243634–3645. [DOI] [PubMed] [Google Scholar]
- Lee B. Y.; Horwitz M. A.; Clemens D. L. Identification, recombinant expression, immuno localization in macrophages, and T-cell responsiveness of the major extracellular proteins of Francisella tularensis. Infect. Immun. 2006, 7474002–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twine S. M.; Mykytczuk N. C.; Petit M. D.; Shen H.; Sjostedt A.; Wayne Conlan J.; Kelly J. F. In vivo proteomic analysis of the intracellular bacterial pathogen, Francisella tularensis, isolated from mouse spleen. Biochem. Biophys. Res. Commun. 2006, 34541621–1633. [DOI] [PubMed] [Google Scholar]
- Havlasova J.; Hernychova L.; Halada P.; Pellantova V.; Krejsek J.; Stulik J.; Macela A.; Jungblut P. R.; Larsson P.; Forsman M. Mapping of immunoreactive antigens of Francisella tularensis live vaccine strain. Proteomics 2002, 27857–867. [DOI] [PubMed] [Google Scholar]
- Hubalek M.; Hernychova L.; Havlasova J.; Kasalova I.; Neubauerova V.; Stulik J.; Macela A.; Lundqvist M.; Larsson P. Towards proteome database of Francisella tularensis. J. Chromatogr., B 2003, 7871149–177. [DOI] [PubMed] [Google Scholar]
- Twine S. M.; Petit M. D.; Fulton K. M.; House R. V.; Conlan J. W. Immunoproteomics analysis of the murine antibody response to vaccination with an improved Francisella tularensis live vaccine strain (LVS). PLoS One 2010, 54e10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twine S. M.; Petit M. D.; Shen H.; Mykytczuk N. C.; Kelly J. F.; Conlan J. W. Immunoproteomic analysis of the murine antibody response to successful and failed immunization with live anti-Francisella vaccines. Biochem. Biophys. Res. Commun. 2006, 3463999–1008. [DOI] [PubMed] [Google Scholar]
- Pavkova I.; Hubalek M.; Zechovska J.; Lenco J.; Stulik J. Francisella tularensis live vaccine strain: proteomic analysis of membrane proteins enriched fraction. Proteomics 2005, 592460–2467. [DOI] [PubMed] [Google Scholar]
- Twine S. M.; Mykytczuk N. C.; Petit M.; Tremblay T. L.; Lanthier P.; Conlan J. W.; Kelly J. F. Francisella tularensis proteome: low levels of ASB-14 facilitate the visualization of membrane proteins in total protein extracts. J. Proteome Res. 2005, 451848–1854. [DOI] [PubMed] [Google Scholar]
- Dresler J.; Klimentova J.; Stulik J. Francisella tularensis membrane complexome by blue native/SDS-PAGE. J. Proteomics 2011, 751257–269. [DOI] [PubMed] [Google Scholar]
- Janovska S.; Pavkova I.; Reichelova M.; Hubaleka M.; Stulik J.; Macela A. Proteomic analysis of antibody response in a case of laboratory-acquired infection with Francisella tularensis subsp. tularensis. Folia Microbiol. 2007, 522194–198. [DOI] [PubMed] [Google Scholar]
- Broms J. E.; Meyer L.; Sun K.; Lavander M.; Sjostedt A. Unique substrates secreted by the type VI secretion system of Francisella tularensis during intramacrophage infection. PLoS One 2012, 711e50473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straskova A.; Pavkova I.; Link M.; Forslund A. L.; Kuoppa K.; Noppa L.; Kroca M.; Fucikova A.; Klimentova J.; Krocova Z.; Forsberg A.; Stulik J. Proteome analysis of an attenuated Francisella tularensis dsbA mutant: identification of potential DsbA substrate proteins. J. Proteome Res. 2009, 8115336–5346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.