Figure 3.
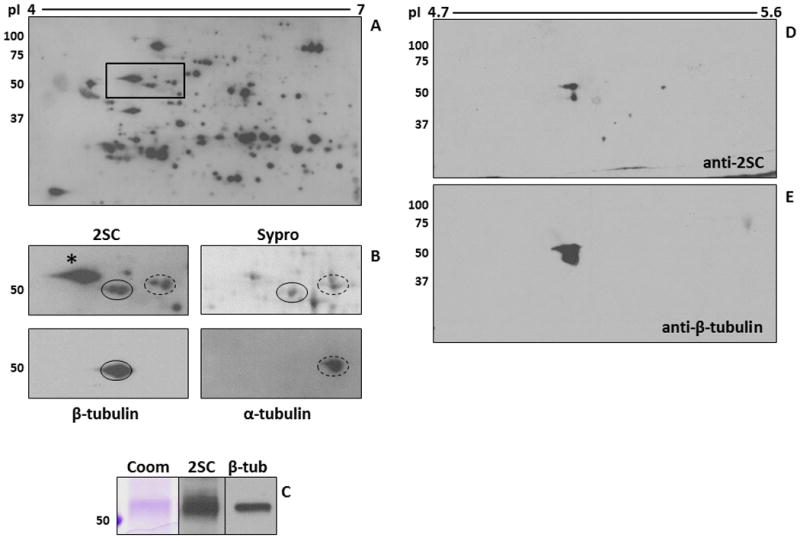
Tubulin succination in 3T3 adipocytes cultured in high glucose medium. (A-B) Protein (500 μg) from 3T3 adipocytes grown in 30 mM glucose was analyzed by 2D gel electrophoresis across a 4-7 pH range. Duplicate gels were either transferred and immunoblotted with an anti-2SC antibody to detect succinated proteins (A, B-2SC), or stained with Sypro Ruby to observe total protein (B-Sypro). After detection of 2SC-modified proteins, the blot was stripped and probed sequentially with antibodies to α-tubulin (B-α-tubulin, dashed line) and β-tubulin (B-β-tubulin, solid line) in order to locate and excise the corresponding 2SC-modified spots in the Sypro Ruby-stained gel for identification (see Table 2). The images shown in panel B correspond to the rectangular area highlighted in panel A. * denotes another ∼50 kDa succinated protein which was identified as Protein Disulfide Isomerase. (C) Protein (500 μg) from 3T3 adipocytes grown in 30 mM glucose was immunoprecipitated with an anti β-tubulin antibody. After treatment of the agarose beads with Laemmli buffer, half of the sample was electrophoresed and stained with Coomassie blue (Coom) to detect immunoprecipitated tubulin. The remainder was electrophoresed and transferred prior to probing with an anti-2SC antibody (2SC), followed by stripping and confirmation of the identity of the protein with an anti β-tubulin antibody (β-tub). (D-E) An adipose tissue extract (200 μg of protein) from db/db mice was immunoprecipitated with an anti β-tubulin antibody and analyzed by 2D gel electrophoresis across a 4.7-5.9 pH range (4.7-5.6 range is shown). The gel was transferred and probed with an anti-2SC antibody (D), followed by stripping and confirmation of protein identity with an anti β-tubulin antibody (E). Molecular masses of marker proteins are indicated on the left-hand side.
