Abstract
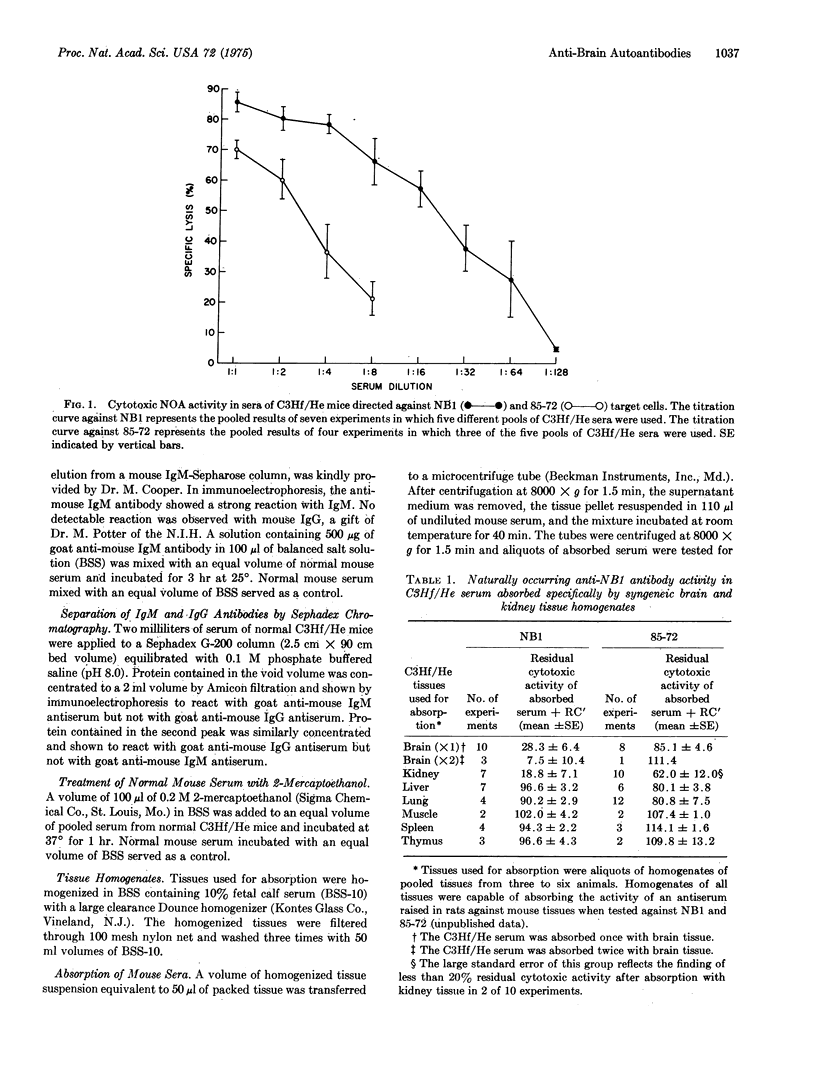
Normal mouse sera contain naturally occurring antibodies that are cytotoxic in the presence of rabbit complement for NB1, a cell line derived from a neuroblastoma adrenal metastasis of a spontaneous ovarian teratoma. The anti-NB1 antibodies can be specifically removed from normal mouse sera by absorption of the sera with homogenized brain tissue of mouse, rat, guinea pig, chicken, and man and by homogenized kidney tissue of mouse and man. The antigen recognized by anti-NB1 naturally occurring autoantibodies, designated mouse brain antigen-2 (MBA-2), is not present on other normal tissues or tumor cell lines tested. MBA-2 is distinct from previously described mouse brain antigens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Widespread natural occurrence of high titers of neutralizing antibodies to a specific class of endogenous mouse type-C virus. Proc Natl Acad Sci U S A. 1974 May;71(5):1957–1961. doi: 10.1073/pnas.71.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeson R., Herschman H. R. Modulation of cell-surface antigens of a murine neuroblastoma. Proc Natl Acad Sci U S A. 1974 Jan;71(1):187–191. doi: 10.1073/pnas.71.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon F. J., Oldstone M. B., Tonietti G. Pathogenesis of immune complex glomerulonephritis of New Zealand mice. J Exp Med. 1971 Sep 1;134(3 Pt 2):65s–71s. [PubMed] [Google Scholar]
- Hellström I., Hellström K. E. Can "blocking" serum factors protect against autoimmunity? Nature. 1972 Dec 22;240(5382):471–473. doi: 10.1038/240471a0. [DOI] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Immunological enhancement as studied by cell culture techniques. Annu Rev Microbiol. 1970;24:373–398. doi: 10.1146/annurev.mi.24.100170.002105. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Aoki T. Immune and natural antibodies to syngeneic murine plasma cell tumors. J Exp Med. 1972 Jul 1;136(1):94–111. doi: 10.1084/jem.136.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Yurconic M., Jr, Hanna M. G., Jr Autogenous immunity to endogenous RNA tumor virus. Radioimmune precipitation assay of mouse serum antibody levels. J Exp Med. 1973 Jul 1;138(1):194–208. doi: 10.1084/jem.138.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. E. Mouse brain antigen detected by rat anti-C1300 antiserum. Nature. 1974 May 3;249(452):71–73. doi: 10.1038/249071a0. [DOI] [PubMed] [Google Scholar]
- Martin W. J. Immune surveillance directed against depressed cellular and viral alloantigens. Cell Immunol. 1975 Jan;15(1):1–10. doi: 10.1016/0008-8749(75)90159-8. [DOI] [PubMed] [Google Scholar]
- Martin W. J., Martin S. E. Naturally occurring cytotoxic anti-tumour antibodies in sera of congenitally athymic (nude) mice. Nature. 1974 Jun 7;249(457):564–565. doi: 10.1038/249564a0. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Kaehler S. L. Antibody to leukemia virus: widespread occurrence in inbred mice. Science. 1974 Sep 6;185(4154):869–871. doi: 10.1126/science.185.4154.869. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Aoki T., Dixon F. J. The antibody response of mice to murine leukemia virus in spontaneous infection: absence of classical immunologic tolerance (AKR mice-complement-fixing antibodies-lymphocytic choriomeningitis virus-immunofluorescence-glomerular deposits of antigen-antibody complexes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):134–138. doi: 10.1073/pnas.69.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygaard J., Povlsen C. O. Is immunological surveillance not a cell-mediated immune function? Transplantation. 1974 Jan 1;17(1):135–136. [PubMed] [Google Scholar]
- Sato H., Boyse E. A., Aoki T., Iritani C., Old L. J. Leukemia-associated transplantation antigens related to murine leukemia virus. The X.1 system: immune response controlled by a locus linked to H-2. J Exp Med. 1973 Sep 1;138(3):593–606. doi: 10.1084/jem.138.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner M. NS-1 (nervous system antigen-1), a glial-cell-specific antigenic component of the surface membrane. Proc Natl Acad Sci U S A. 1974 May;71(5):1795–1799. doi: 10.1073/pnas.71.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid M., Boyse E. A., Carswell E. A., Old L. J. Serologically demonstrable alloantigens of mouse epidermal cells. J Exp Med. 1972 Apr 1;135(4):938–955. doi: 10.1084/jem.135.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Mellors R. C. Natural thymocytotoxic autoantibody and reactive antigen in New Zealand black and other mice. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1412–1415. doi: 10.1073/pnas.68.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Boyse E. A. Surface alloantigens of plasma cells. J Exp Med. 1970 Jun 1;131(6):1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]


