Table 1. Effect of the porphyrin ligand on the stereoselective metalloradical C–H alkylation of α-methoxycarbonyl-α-diazosulfone 1a catalyzed by [Co(D 2-Por*)] a .
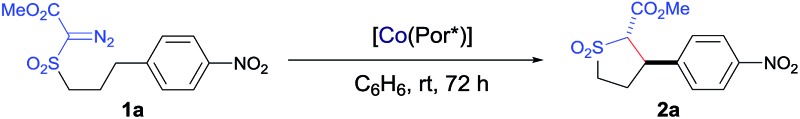
| ||||
| Entry | Catalyst (loading) | Yield b (%) | dr c | ee d (%) |
| 1 | [Co(TPP)] (120 mol%) | NR e | — | — |
| 2 | [Co(P1)] (2 mol%) | 83 | 95 : 5 | –24 |
| 3 | [Co(P2)] (2 mol%) | 63 | 96 : 4 | 91 |
| 4 | [Co(P3)] (2 mol%) | 92 | 96 : 4 | 92 |
| ||||
aReactions were carried out at room temperature for 72 h in a one-time fashion without slow addition of the diazo reagent using [Co(Por)] under N2.
bIsolated yields.
cThe trans : cis diastereomeric ratio determined by 1H-NMR.
dEnantiomeric excess determined by chiral HPLC.
eNo reaction.
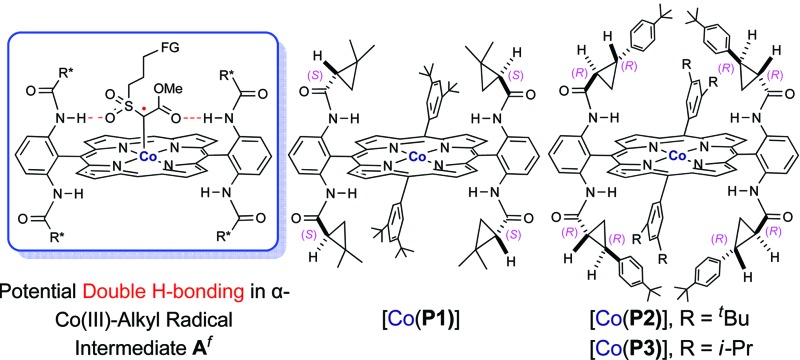
fFor clarity, the other two meso-groups of the porphyrin are omitted.
