Table 4. Diastereoselective transformations of sulfolanes with construction of quaternary carbon stereocenters a .
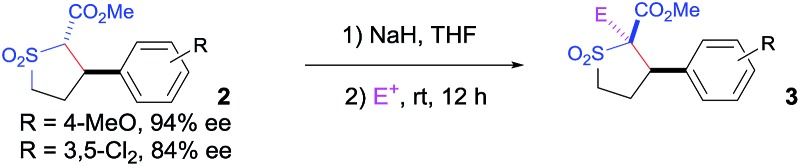
| |||||
| Entry | Electrophile | Product | Yield (%) | dr | ee (%) |
| 1 b | Selectfluor |
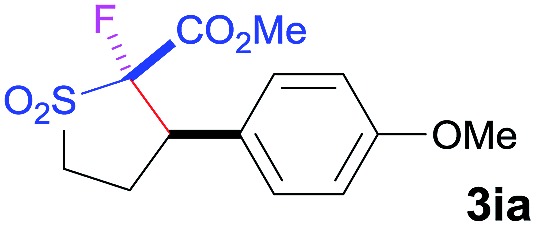
|
92 | 96 : 4 | 94 |
| 2 b | Selectfluor |
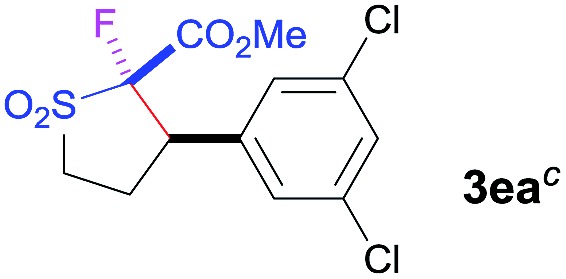
|
89 | 96 : 4 | 84 |
| 3 | NCS |
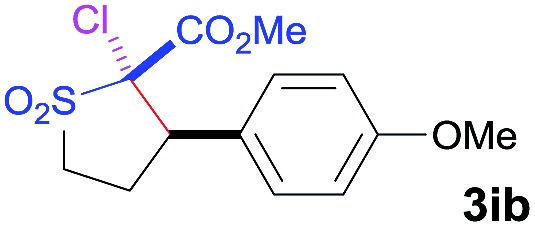
|
92 | 97 : 3 | 93 |
| 4 | MeI |
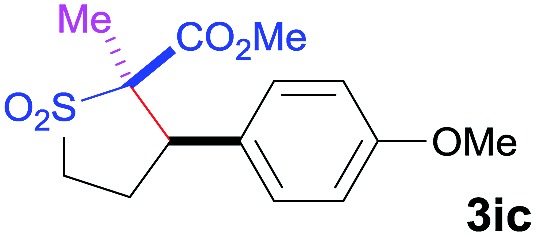
|
91 | 8 : 92 | 93 |
| 5 d | Ethyl acrylate |
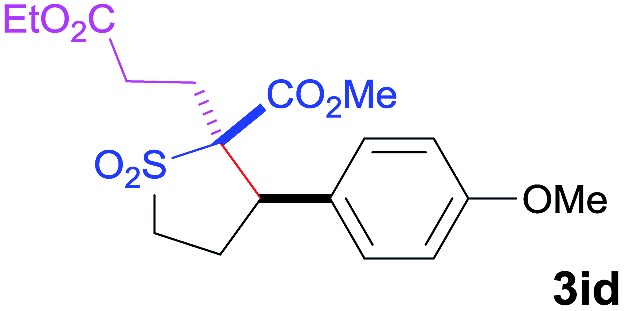
|
60 | 4 : 96 | 93 |
aCompound 2 was treated with 1.2 equiv. of NaH in THF at room temperature, followed by the addition of 1.1 equiv. of electrophile and the subsequent stirring of the reaction mixture for 12 h; isolated yields; the trans : cis diastereomeric ratios were determined by 1H-NMR; enantiomeric excesses were determined by chiral HPLC.
bTHF/DMF (2 : 1) used as solvent.
c[2R,3R] absolute configuration determined by anomalous-dispersion effects in X-ray diffraction measurements on a crystal.
dThe reaction was stirred for 3 h.
