Figure 8. Diagrams indicating the location of the critical threonine 389 residue in STIM1.
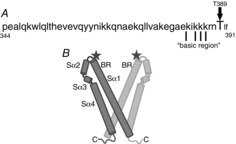
A, amino acid sequence of the region of STIM1 that forms the ‘second coiled-coil’ domain that is thought to be involved in the activation of Orai channels, and the position of the T389 residue in this sequence. Indicated are the series of critical lysine residues within the so-called ‘basic domain’, mutation of which blocks activation of both CRAC and ARC channels (see text for details). B, diagram indicating the location of the T389 residue (‘star’) at the apex of the proposed V-shape cleft formed by the predicted crystal structure of the CAD/SOAR region of a STIM1 dimer (BR, basic region; Sα1-4, sequential predicted α-helical domains). Based on the structures reported by Yang et al. (2012).
