Figure 3. Combination of uraemic toxins increases ILK activity in EA.hy926 cells.
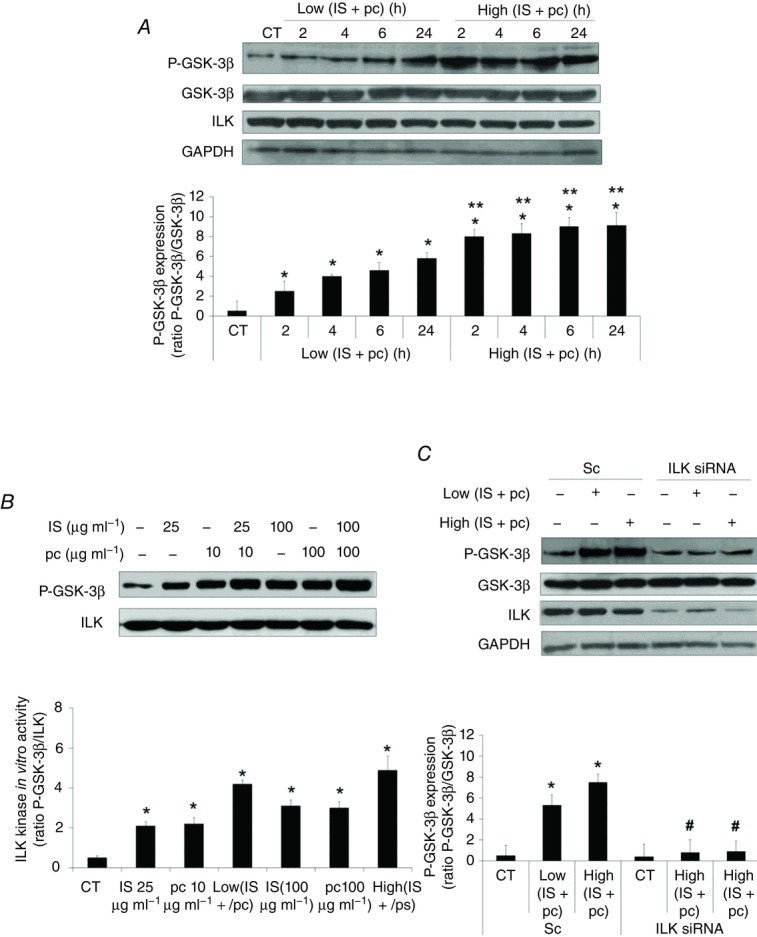
A, cells were incubated in medium supplemented with 2.5% normal serum (NS) plus a combination of low concentrations of indoxyl sulfate (IS; 25 μg ml−1) and p-cresol (pc; 10 μg ml−1) (Low IS + pc) or plus a combination of high concentrations of IS (100 μg ml−1) and pc (100 μg ml−1) (High IS + pc) for different times. Representative Western blots of phosphorylated GSK-3β in the serine-9 residue (P-GSK-3β) or ILK are shown. Total GSK-3β or GAPDH levels were determined as endogenous control. B, cells were incubated in medium supplemented with 2.5% NS plus IS (25 or 100 μg ml−1), pc (10 or 100 μg ml−1), Low (IS + pc) or High (IS + pc), for 24 h. In vitro kinase activity of ILK was determined in cell lysates, by immunoprecipitation of ILK followed by incubation with a fixed amount of exogenous GSK-3 protein-fusion as substrate. Levels of xogenous GSK-3 protein phosphorylation in the serine-9 residue (P-GSK-3β) were measured by Western blot and equal ILK loading was confirmed. C, cells were depleted of ILK with specific siRNA (100 nm) and treated afterwards as in A for 24 h. Representative Western blots of phosphorylated GSK-3β in the serine-9 residue (P-GSK-3β) or ILK are shown. Total GSK-3β or GAPDH levels were determined as endogenous control. Bars represent the normalized densitometric analysis of the blots against the endogenous control (A and C) or ILK (B) values. All values are represented as mean ± SEM of six independent experiments. *P < 0.05 vs. control (CT; 2.5% NS, 24 h), **P < 0.05 vs. Low (IS + pc), #P < 0.05 vs. Sc.
