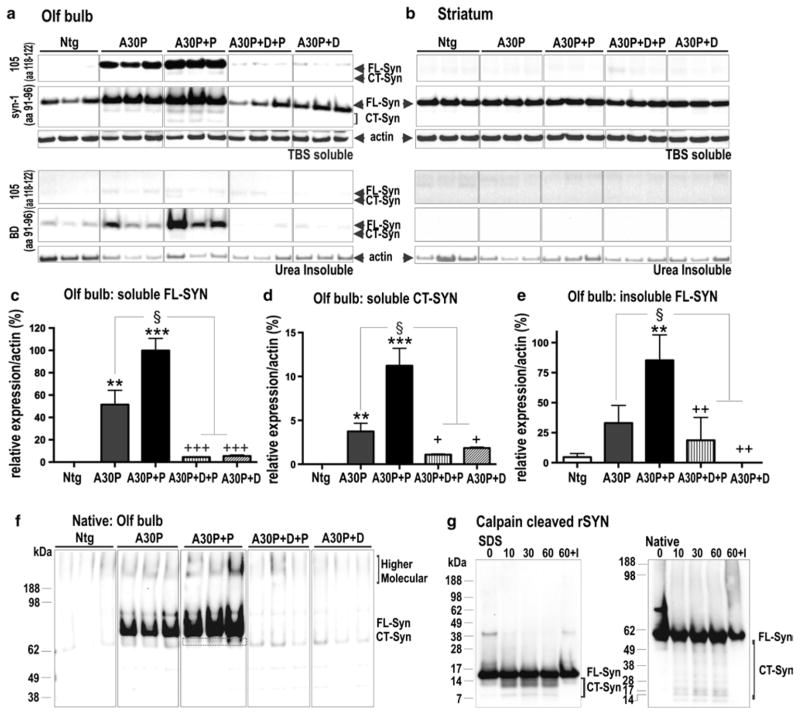
Fig. 3.
Biochemical characterization of α-syn pattern reveals paraquat-dependent increase in oligomeric and CT α-syn. a, b Immunoblots from western blot analysis of OB and striatal extracts from non-transgenic (WT), A30P and paraquat-treated A30P+P and dox-treated control (A30P+D+P, A30P+D) mice. Antibodies that either recognized full-length (FL) and C-terminal truncated (CT) human α-syn (105) or total (murine and human) α-syn (syn-1) showed that human α-syn was only present in OB tissue. c Quantification of immunoblots showed that A30P+P mice present an increase of TBS-soluble FL α-syn and d CT α-syn. A series of ultracentrifugation and extraction steps were used to obtain urea-insoluble fractions of FL α-syn and CT α-syn. A faint CT α-syn signal in urea extracts was solely present in A30P+P mice. e WT and dox-treated (A30P+D+P, A30P+D) control mice only showed minor levels of urea-insoluble α-syn. In contrast, quantification of urea-fractions of A30P+P mice revealed strong increase of insoluble α-syn when compared to untreated A30P mice. f Native PAGE. α-Syn at ~60 to 70 kDa and higher molecular species accumulate in A30P+P mice. Bands below the main species may present truncated α-syn or cross-reaction of 105 antibody. g Left SDS-PAGE/Western blot (antibody syn-1) analysis of recombinant α-syn digested with calpain-1 for 0, 10, 30 and 60 min, and with recombinant α-syn incubated with calpain-1 and calpeptin, an inhibitor (I). Right same samples were subjected to native PAGE/ Western blot (antibody syn-1) showing main ~55 to 60 kDa monomeric α-syn (FL-Syn) and below calpain-1 cleaved α-syn fragments (CT-Syn). Immunoblots are highlighted by separating boxes for clarity of display. The data are expressed as mean ± SEM. **p < 0.01, ***p < 0.001 vs. WT; §p < 0.05 vs. A30P; +p < 0.05, ++p < 0.01, +++p < 0.001 vs. A30P+P