FIG 7.
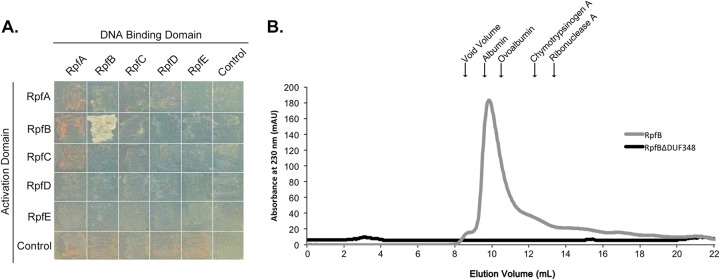
RpfB forms dimers. (A) Yeast two-hybrid analysis of interactions between Rpf proteins. Pictures were taken after 2 days of incubation at 30°C. Growth on selective medium indicates an interaction between bait (DNA-binding domain)- and prey (activation domain)-associated proteins. (B) Purified RpfB and RpfBΔDUF348 were separated on a Sephadex 75 gel filtration column. The peak at 9.4 ml represents likely RpfB dimers. RpfBΔDUF348 dimers were expected to elute at 12 ml. The molecular mass and elution volume, respectively, of each of the standards indicated above the figure are as follows: albumin, 67 kDa and 9.6 ml; ovalbumin, 43 kDa and 10.4 ml; chymotrypsinogen A, 25 kDa and 12.4 ml; and RNase A, 13.7 kDa and 13.3 ml.
