Abstract
The bulk of bacterial protein secretion occurs through the conserved SecY translocation channel that is powered by SecA-dependent ATP hydrolysis. Many Gram-positive bacteria, including the human pathogen Listeria monocytogenes, possess an additional nonessential specialized ATPase, SecA2. SecA2-dependent secretion is required for normal cell morphology and virulence in L. monocytogenes; however, the mechanism of export via this pathway is poorly understood. L. monocytogenes secA2 mutants form rough colonies, have septation defects, are impaired for swarming motility, and form small plaques in tissue culture cells. In this study, 70 spontaneous mutants were isolated that restored swarming motility to L. monocytogenes secA2 mutants. Most of the mutants had smooth colony morphology and septated normally, but all were lysozyme sensitive. Five representative mutants were subjected to whole-genome sequencing. Four of the five had mutations in proteins encoded by the lmo2769 operon that conferred lysozyme sensitivity and increased swarming but did not rescue virulence defects. A point mutation in secY was identified that conferred smooth colony morphology to secA2 mutants, restored wild-type plaque formation, and increased virulence in mice. This secY mutation resembled a prl suppressor known to expand the repertoire of proteins secreted through the SecY translocation complex. Accordingly, the ΔsecA2prlA1 mutant showed wild-type secretion levels of P60, an established SecA2-dependent secreted autolysin. Although the prl mutation largely suppressed almost all of the measurable SecA2-dependent traits, the ΔsecA2prlA1 mutant was still less virulent in vivo than the wild-type strain, suggesting that SecA2 function was still required for pathogenesis.
INTRODUCTION
The essential general secretory (Sec) pathway is responsible for exporting the majority of secreted proteins across the bacterial cell membrane (1–3). Much of what we know about this pathway was discovered in Escherichia coli by using genetic screens to identify loss-of-function mutations in components of the Sec system. This led to the identification of components of the SecYEG complex that forms the translocation channel and the SecA ATPase that binds to signal sequences to drive the export of precursor proteins across the channel (3–9). In contrast to loss-of-function sec mutations, prl alleles are gain-of-function mutations, which expand the repertoire of substrates being exported across the SecYEG channel (6–8, 10, 11). The most dominant prl variants are in secY, known as prlA mutations, which allow for the secretion of proteins with altered signal sequence (7, 9, 11) without significantly altering the secretion of other proteins (6, 12).
In addition to the canonical SecA, an accessory cytosolic ATPase, SecA2, has been identified in Mycobacterium and a subset of other Gram-positive bacteria. Unlike SecA, SecA2 is not essential for cell viability but is required for virulence in some pathogenic bacteria (13–15). SecA1 and SecA2 are homologous but are not interchangeable (16). There are two types of SecA2 secretion pathways; some bacteria have an additional SecY homolog, which functions together with SecA2 to form a SecA2-SecY2 system. This system has been extensively studied in streptococci and is highly specialized to export glycosylated proteins that are incompatible with the canonical Sec system (13, 17–20). Other bacteria contain a SecA2-only system, which is thought to interact with the canonical SecYEG channel (16, 21, 22). The repertoire of proteins that require the accessory SecA2 for secretion is relatively small and varies between organisms (13, 17).
Listeria monocytogenes is a saprophytic Gram-positive, facultative intracellular pathogen capable of causing food-borne listeriosis in humans (23, 24). Spontaneous rough mutants of L. monocytogenes commonly arise on solid media, and many of these contain mutations in secA2 (25–27). Mutants in secA2 exhibit a chaining phenotype and are approximately 1,000-fold less virulent in animal models of infection (14, 25, 28). These mutants secrete a reduced subset of proteins, including two major autolysins, P60 (CwhA) and NamA (MurA), that contribute to septation and pathogenesis (14, 29–32). To better understand SecA2-dependent protein secretion and the contribution that SecA2 makes to L. monocytogenes pathogenesis, we undertook a suppressor analysis of a ΔsecA2 mutant. The primary finding reported in this communication is that a prlA mutation rescued most of the defects associated with a secA2 mutation, although it did not lead to full restoration of virulence.
MATERIALS AND METHODS
Ethics statement.
This study was carried out in strict accordance with the recommendations specified in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (33). All protocols were reviewed and approved by the Animal Care and Use Committee at the University of California Berkeley (Master Animal Use Protocol R235-0813B).
Bacterial strains and growth conditions.
All L. monocytogenes strains used and generated in this study were in the 10403S background and are listed in the supplemental material (see Table S1 in the supplemental material). The ΔsecA2 strain previously characterized (14, 25) served as a parent strain for generating motility revertants. Unless otherwise stated, all L. monocytogenes strains were grown in brain heart infusion medium (BHI; Difco, Detroit, MI). All E. coli strains used for generating in-frame deletion and complement constructs were grown in Luria-Bertani (LB) medium (Difco, Detroit, MI).
Swarming motility.
A single colony of the ΔsecA2 strain was used to generate a single motility revertant by stab-inoculating semisolid LB 0.4% agar at 30°C for 5 days. Revertant strains were grown overnight in BHI broth at 30°C without shaking; 1 μl of culture was then incorporated into a semisolid LB agar, and the swarming area was evaluated using ImageJ (http://rsbweb.nih.gov/ij/) following incubation at 30°C for 48 h.
L2 plaque assay.
Plaque assays using murine L2 fibroblasts were performed as previously described (34). Briefly, overnight 30°C static cultures of L. monocytogenes were allowed to infect monolayers of L2 cells for 1 h. Cells were washed and overlaid with 0.7% agarose in Dulbecco's modified Eagle's medium (DMEM; Gibco/Invitrogen, California) containing 10 μg/ml gentamicin (GM). After 3 days at 37°C, plaques were overlaid with 2 ml of 0.7% agarose in DMEM with GM and 0.3% neutral red (Sigma-Aldrich). Monolayers were stained overnight, and plaque size was evaluated using ImageJ.
DNA isolation and sequencing.
Genomic DNA was isolated from stationary-phase cultures of L. monocytogenes using the MasterPure DNA purification kit (Epicentre). DNA was then fragmented using Covaris S22 (Covaris Inc.). Libraries were constructed using Apollo 324 (IntegenX Inc.), PCR amplified, and multiplexed at the Vincent J. Coates Genomics Sequencing Laboratory at UC Berkeley. The resulting libraries were sequenced using single-end reads with a HiSeq 2000 Illumina platform. Sequence data were aligned to the L. monocytogenes 10403S reference genome (GenBank accession number CP002002.1) using Bowtie 2 (35), and single-nucleotide polymorphisms (SNPs) were identified using SAM tools (36). Approximately 93% of all reads aligned to the reference genome, resulting in >50× coverage.
In-frame deletion and complementation constructs.
In-frame deletion mutants of lmo2769 and lmo1721 were constructed by splice overlap extension and introduced by allelic exchange using pKSV7 vector and primers JD17 to JD20 and JD30 to JD33, respectively (see Table S2 in the supplemental material), as previously described (37). The pPL2 integration vector was used to complement Δlmo2769 mutants and the R57 revertant strain with the lmo2769-2767 operon and all the endogenous regulatory sequences using the JD46 and JD47 primers (see Table S2 in the supplemental material) as previously described (38).
Transductions.
Transductions of the lmo2768::Tn and lmo2637::Tn donor strains were generated using phage U153 and erythromycin as a selection marker, as previously described (39). Strain R57 containing the prlA1 allele was used as the recipient strain for lmo2637::Tn, and subsequent recipient strains of secY mutation were confirmed by Sanger sequencing (Elim Biopharmaceuticals, Hayward, CA).
In vivo mouse infections.
CD1 [Crl:CD1(ICR)] mice (Charles River Laboratories) were injected intravenously with 1 × 105 CFU of wild-type (WT) or mutant L. monocytogenes strains. Spleens and livers were harvested after 48 h, and bacterial burdens were evaluated as previously described (40).
Disk diffusion assay.
A total of 3 × 108 stationary-phase L. monocytogenes cells were plated on BHI agar plates. Sterile Whatman paper discs (7 mm in diameter) containing 1 mg of chicken egg white lysozyme (Sigma), 10 μg of vancomycin, or 120 μg of penicillin in a 10-μl volume were added to plates. The area of growth inhibition around each disc was measured using ImageJ following 24 h of incubation at 37°C.
Microscopy.
Phase-contrast microscopy was performed using stationary BHI culture at 37°C. Fluorescence microscopy was conducted using an Olympus IX81 TIRF microscope on mid-log cells stained with SYTO9 green fluorescent stain (Invitrogen).
Western blotting.
Secreted proteins from mid-log phase (5 h) or for the time course experiment from 1-h, 2-h, and 3-h LB culture supernatants were precipitated with 10% trichloroacetic acid (TCA), as previously described (41), and solubilized in NuPAGE LDS buffer (Invitrogen) containing 5% β-mercaptoethanol at a volume adjusted to an optical density at 600 nm (OD600) for each strain. Samples were fractionated by SDS-PAGE, transferred to a polyvinylidene difluoride (PVDF) membrane for immunoblotting with polyclonal anti-P60 and anti-LLO antibodies, and quantified using the Odyssey infrared imaging system (LI-COR Biosciences).
RESULTS
Isolation and initial characterization of ΔsecA2 suppressor mutants.
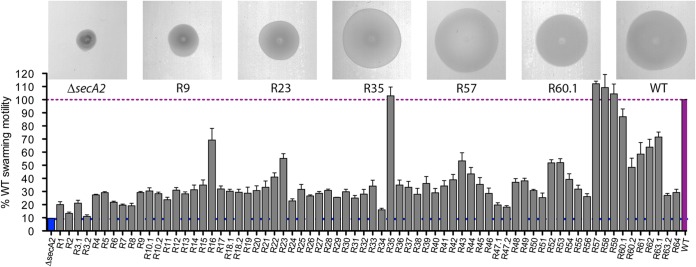
The inability of bacteria to properly septate during cell division can negatively influence swarming efficiency in semisolid media (42–44). Indeed, the filamentous nature of the L. monocytogenes secA2 mutant led to a 92% reduction in swarming motility compared to that of the wild-type (WT) 10403S strain (Fig. 1). Seventy spontaneous, independent ΔsecA2 suppressor mutants were generated by identifying swarming bacteria that appeared after 5 days of incubation at 30°C in semisolid LB agar. Prolonged incubation for isolation of motility revertants was used based on the original isolation of spontaneous rough colonies, which facilitated the identification of secA2 in Listeria (25). These suppressor mutants displayed a wide range of swarming phenotypes (11 to 112%) compared to the WT strain (Fig. 1). Motility revertants were further characterized based on their colony and microscopic appearance (see Table S3 in the supplemental material). The majority of suppressor mutants (64%) reverted from a rough to a smooth colony morphology. Rough colony morphology correlated with chaining, with less chaining observed for isolates forming smooth colonies. Whereas the WT (45–47) and the secA2 mutants of L. monocytogenes are lysozyme resistant, all 70 suppressor mutants were susceptible to lysozyme (see Table S3 in the supplemental material).
FIG 1.
Swarming motility of suppressor mutants. Motility assessed on semisolid LB agar following incubation at 30°C after 48 h. Motility is expressed as a percentage of the swarming area of the WT strain seeded on the same plate. Error bars represent standard deviations of the mean zone ratios from three independent experiments. Images show motility zones for the suppressor mutants indicated.
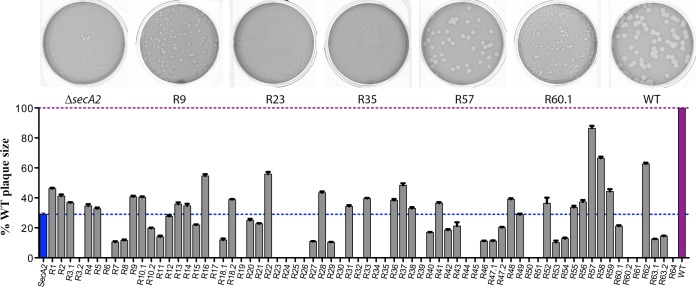
A critical aspect of L. monocytogenes pathogenesis is the capacity to spread from cell to cell and form plaques in monolayers of tissue culture cells, which correlates well with mouse virulence (48). The secA2 mutant forms plaque sizes that are approximately 30% of those of the WT (Fig. 2) (14). The ΔsecA2 swarming suppressor mutants varied greatly in their ability to form plaques, with sizes ranging from 0% to 87% of those of the WT (Fig. 2). There was no significant correlation between chaining phenotype and plaque size, suggesting that chaining alone does not directly influence cell-to-cell spread, as measured by the plaque assay.
FIG 2.
Plaque size of secA2 suppressor mutants. L2 fibroblasts were infected with secA2 suppressor mutants, and mean plaque size was measured 3 days postinfection. Plaque area of each mutant is shown as a percentage compared to that of the WT strain. Error bars represent standard deviations of the mean zone ratios from three independent experiments. Images show plaques for the suppressor mutants indicated.
Whole-genome sequencing of five ΔsecA2 suppressor mutants.
To identify mutations responsible for the observed phenotypes, five strains, R9, R23, R35, R57, and R60.1, were selected for further analysis (Table 1). Whole-genome sequencing revealed a number of single-nucleotide polymorphisms (SNPs) that differed from the WT strain and confirmed that each lacked the secA2 gene (Table 2). Most revertants encoded three to four SNPs, while revertant R35 was an outlier with 13 SNPs, one of which mapped to lmo1403, a gene encoding the DNA mismatch repair protein MutS. We hypothesize that inactivation of mutS led to a hypermutation phenotype (49). The presence of multiple mutations in all suppressors was likely a consequence of extended incubation that allowed for acquisition of multiple mutations, which collectively promoted swarming.
TABLE 1.
Strains selected for sequencing and their phenotypes
| Strain | Colony | Chainingb | % motility (±SD)c | % plaque area (±SD)d |
|---|---|---|---|---|
| R9 | Rough | +++ | 29 (±3) | 41 (±9) |
| R23 | Smooth | + | 55 (±13) | 0 |
| R35 | Smooth | ++ | 102 (±26) | 0 |
| R57 | Smooth | + | 112 (±15) | 87 (±13) |
| R60.1 | Smooth | ++ | 87 (±23) | 21 (±6) |
| ΔsecA2 straina | Rough | ++++ | 9 (±2) | 29 (±9) |
The parent ΔsecA2 strain was included as a reference strain.
Chaining score interpretation by phase microscopy. +, single cells and chains of up to 3 cells/field at ×40 magnification; ++, single cells and chains of up to 4 cells/field at ×40 magnification; +++, chains of up to 6 cells/field at ×40 magnification; ++++, chains of >6 cells/field at ×40 magnification.
Expressed as an average percentage (standard deviation) of the swarming area normalized to that of the WT strain on 0.4% LB agar after 48h at 30°C.
Expressed as an average percentage (standard deviation) of the plaque area compared to that of the WT strain.
TABLE 2.
Single-nucleotide polymorphisms of strains sequenced
| Strain | Positiona | Referencea | Alteration | Change | Lmo no. | Gene name | Encoded protein or function |
|---|---|---|---|---|---|---|---|
| R9 | 1104029 | C | T | A141V | lmo1087 | Ribitol-5-phosphate 2-dehydrogenase | |
| 1921584 | T | C | V195 silent | lmo1892 | pbpA | Penicillin-binding protein 1 | |
| 2812912 | A | G | 48 bp upstream of lmo2769 transcription start site | lmo2769 | Antibiotic transport system ATP-binding protein | ||
| R23 | 714441 | A | G | E28G | lmo0699 | fliM | Flagellar motor switch |
| 2734020 | G | C | G122 silent | lmo2694 | Lysine decarboxylase | ||
| 2811145 | G | T | T169 stop codon | lmo2768 | Membrane protein (permease) | ||
| R35 | 728791 | G | A | A340T | lmo0714 | fliG | Flagellar motor switch protein G |
| 736353 | A | G | E176G | lmo0723 | Methyl-accepting chemotaxis protein | ||
| 1102328 | C | T | P499S | lmo1085 | Similar to teichoic acid biosynthesis protein B | ||
| 1191185 | T | C | I486T | lmo1208 | cbiP | Cobyric acid synthase | |
| 1264242 | CTTTTTTTT | CTTTTTTT | 58 bp upstream of lmo1281 transcription start site | lmo1281 | Hydroxybenzoyl coenzyme A thioesterase | ||
| 1343106 | G | A | R232H | lmo1360 | folD | Methylenetetrahydrofolate dehydrogenase/cyclohydrolase | |
| 1390074 | GAAAAAAAA | GAAAAAAA | I309S, premature stop at codon 319 | lmo1403 | mutS | DNA mismatch repair protein MutS | |
| 1932076 | A | G | S247A | lmo1901 | panC | Pantothenate synthetases | |
| 2137030 | A | G | L219W | lmo2100 | Similar to transcriptional regulator (GntR family) | ||
| 2323057 | G | A | R264C | lmo2278 | lysA | l-Alanoyl-d-glutamate peptidase | |
| 2447109 | CAAAAAAA | CAAAAAAAA | A25C, premature stop at codon 49 | lmo2421 | Similar to two-component sensor histidine kinase | ||
| 2468643 | CTTTTTTTT | CTTTTTTT | A46Q, premature stop at codon 62 | lmo2444 | Similar to glycosidase | ||
| 2812179 | ATTTTTTT | ATTTTTTTT | I229N, premature stop at codon 236 | lmo2769 | Antibiotic transport system ATP-binding protein | ||
| R57 | 1738627 | TAA | TAAA | L780F, premature stop at codon 791 | lmo1721 | lacR | Sigma-54 interaction domain-containing protein |
| 2658496 | C | T | G408R | lmo2612 | secY | Protein translocase subunit SecY | |
| 2812179 | ATTTTTTT | ATTTTTTTT | I229N, premature stop at codon 236 | lmo2769 | Antibiotic transport system ATP-binding protein | ||
| R60.1 | 311857 | CAAAAAA | CAAAAAAA | G109R, premature stop at codon 279 | lmo0290 | walI | Similar to B. subtilis YycI protein |
| 2542503 | C | T | D559N | lmo2510 | secA | Protein translocase subunit SecA | |
| 2568428 | T | CA | V211G, premature stop at codon 223 | lmo2537 | mnaA | UDP-N-acetylglucosamine 2-epimerase |
Reference genome, CP002002.1.
Two revertants, R23 and R35, contained SNPs in genes encoding the flagellar motor switch proteins FliM and FliG, respectively (Table 2). Both of these proteins function in controlling the direction of flagellar rotation (50–53), and single point mutations in FliM have been shown to restore swarming motility in a chemotaxis-deficient mutant of Salmonella enterica (52). We hypothesize that SNPs in these genes led to enhanced swarming motility of the corresponding revertants by direct manipulation of the flagellar motor. However, neither of these suppressor mutants formed plaques in tissue culture cells and therefore were not subjected to further analysis.
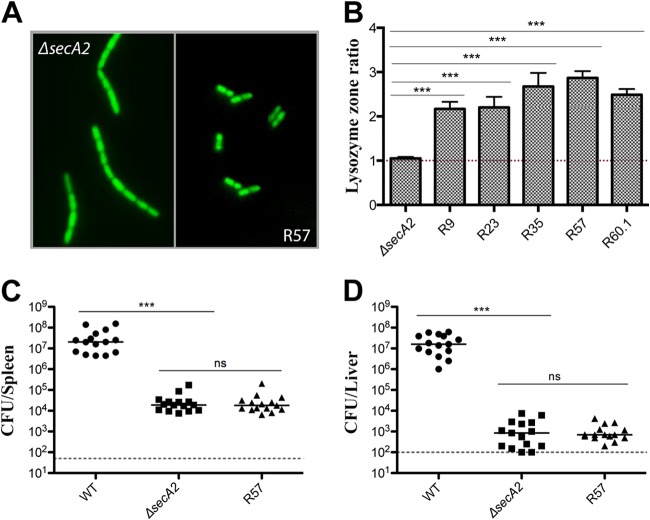
All suppressor mutants were significantly more susceptible to lysozyme than the WT or the parent secA2 mutant strain (Fig. 3B; see also Table S3 in the supplemental material). Susceptibility to cell wall-acting antibiotics was less prominent but still evident for R23, R35, and R60.1 (see Fig. S1A and B in the supplemental material). Additionally, four out of five suppressors had mutations in the lmo2769 operon, previously shown to be required for lysozyme resistance (46). Lastly, mutant R60.1 contained a mutation in walI, a regulator of the WalRK two-component system required for autolysin regulation and lysozyme resistance in L. monocytogenes (46).
FIG 3.
Characterization of the R57 revertant. (A) Fluorescence microscopy of the ΔsecA2 and R57 strains. Mid-exponential-phase cells were stained with SYTO9 green. (B) Disk diffusion susceptibility to 1 mg of lysozyme expressed as a ratio of the WT, where a ratio of >1 indicates increased susceptibility to lysozyme. Error bars represent standard deviations of the mean zone ratios. Student's t test was used to analyze statistical significance: ***, P < 0.0001; ns, P ≥ 0.05. Bacterial burdens in spleens (C) and livers (D) 48 h postinfection with 1 × 105 CFU in CD1 mice. The dashed line represents the limit of detection. Results show CFU from three independent experiments. Statistical significance was evaluated using a Mann-Whitney test. ***, P < 0.0001; ns, P ≥ 0.05.
Most notably, mutants R57 and R60.1 contained SNPs in genes encoding two essential components of the canonical Sec pathway, SecY and SecA, respectively. These mutations resulted in amino acid substitutions leading to altered protein function, and the SNP in secY is analyzed in detail below. The identification of mutations in secY and secA suggests a link between SecA2 and the general Sec pathway in L. monocytogenes.
Improved swarming motility and lack of chaining did not improve cell-to-cell spread in most of the mutants, with the exception of R57. This mutant showed a complete loss of chaining (Fig. 3A) and restored cell-to-cell spread to 87% of the WT strain (Fig. 1). Based on the increased plaque size, we hypothesized that this strain would show increased virulence in vivo. However, upon intravenous infection of mice, the R57 mutant was just as attenuated as the ΔsecA2 parental strain (Fig. 3C and D). The lack of even a partial rescue of virulence was somewhat surprising and suggested that either the secA2 mutation cannot be overcome in vivo or that the combination of SNPs resulted in virulence suppression. Because of these prominent phenotypes and a possible link with the SecYEG translocon, R57 was chosen for further analysis.
Mutations in the lmo2769 operon resulted in lysozyme susceptibility and increased swarming motility but did not contribute to increased plaque formation.
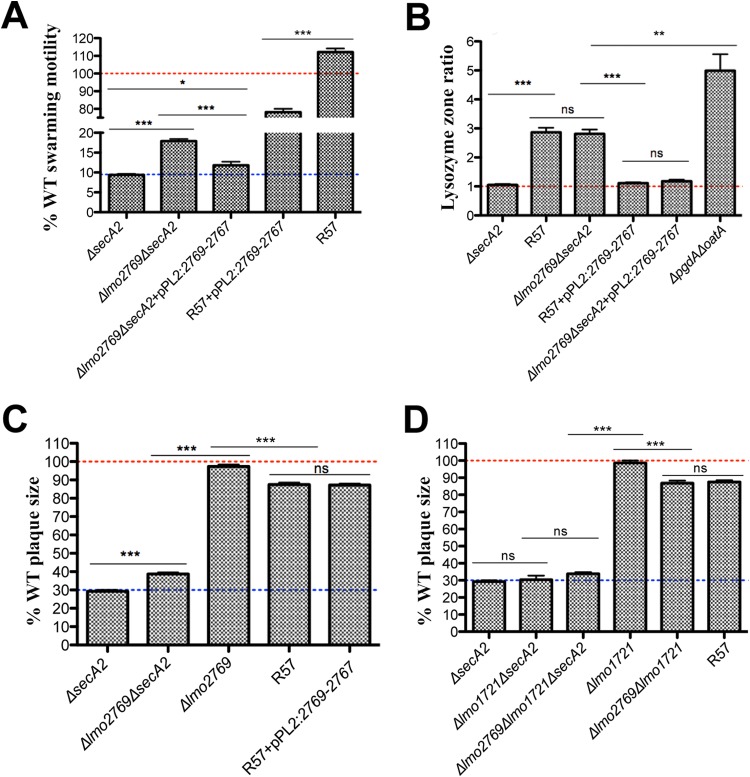
Four out of five sequenced revertants had SNPs in the lmo2769 operon (Table 2). The first gene of this uncharacterized operon, lmo2769, encodes an ATP-binding protein, followed by an ABC transporter permease and a membrane protein encoded by lmo2768 and lmo2767, respectively. The function of the ABC transporter is unknown; however, it has been implicated in lysozyme sensitivity (46). It seemed probable that mutations in this operon, in addition to contributing to lysozyme susceptibility, also enhanced swarming motility of ΔsecA2 revertants. Indeed the swarming motility of the Δlmo2769 ΔsecA2 mutant was twice that of the parent ΔsecA2 strain (Fig. 4A). The Δlmo2769 mutants were also significantly more susceptible to lysozyme than were the WT or ΔsecA2 strains (Fig. 4B; see also Fig. S1 in the supplemental material) (46). There was no change in susceptibility to other cell wall-acting agents tested (see Fig. S1 in the supplemental material); however, lmo2768::Tn has been shown to be more susceptible to cationic antimicrobial peptides (CAMPs) and cefuroxime (46). The increase in swarming motility and lysozyme susceptibility was diminished by complementing either the Δlmo2769 ΔsecA2 double mutant or R57 with the lmo2769 operon (Fig. 4A and B).
FIG 4.
Characterization of an lmo2769 mutant in a ΔsecA2 background. (A) Swarming motility of mutants and strains complemented with the lmo2767 operon was assessed on semisolid LB agar following incubation at 30°C after 48 h. Motility is expressed as a percentage of the swarming area of the WT strain and set at 100% (represented by a red dotted line). Error bars represent standard deviations of the mean zone ratios from three independent experiments. (B) Disk diffusion susceptibility to 1 mg of lysozyme expressed as a ratio of the WT. Error bars represent standard deviations of the mean zone ratios. (C and D) Plaque area of each mutant is shown as a percentage of that of the WT strain, set at 100% (represented by a red dotted line). Error bars represent standard deviations of the mean plaque size ratios from three independent experiments. Student's t test was used to analyze statistical significance: ***, P < 0.0001; **, P < 0.01; *, P < 0.05; and ns, P ≥ 0.05.
Lysozyme susceptibility caused by the disruption of the lmo2769 operon had a negligible impact on virulence (Fig. 4C; see also Fig. S2A and B in the supplemental material). Plaque size of the ΔsecA2 Δlmo2769 double mutant increased 10% from that of the ΔsecA2 strain; however, complementation of R57 with the lmo2769-lmo2767 operon had no effect on plaque formation of the revertant (Fig. 4C). We concluded that disruption of lmo2769 led to a cell wall defect that increased swarming motility of the mutant but was not responsible for the increased plaque size in the originally isolated R57 suppressor strain.
Deletion of lmo1721, a gene harboring the second SNP identified in R57, had a suppressive effect on the swarming motility of the ΔsecA2 strain and the ΔsecA2 Δlmo2769 double mutant (see Fig. S3A in the supplemental material) and had no effect on lysozyme susceptibility (see Fig. S1F in the supplemental material). This gene encodes the transcriptional regulator LacR, which has been suggested to suppress virulence in L. monocytogenes in response to cellobiose (54, 55) in addition to regulating the phosphotransferase system (56). Plaque formation was unaffected by in-frame deletion of the lmo1721 gene alone or in combination with a secA2 deletion (Fig. 4D) but had a suppressive effect on the Δlmo1721 Δlmo2769 double mutant, suggesting that the combination of the two mutations negatively influences virulence when SecA2 is undisturbed. Collectively, these observations suggest that the SNP in lmo1721 did not contribute to the revertant phenotype.
The secY mutation significantly improved virulence and swarming motility of the ΔsecA2 strain.
The third SNP identified in the R57 revertant was in lmo2612, encoding SecY, which resulted in the G408R amino acid substitution. The G408 residue corresponded to V411 when aligned to the E. coli SecY and mapped to the last transmembrane segment of the protein. Mutations in this region of E. coli SecY, such as L407R and I408N, have been described as protein localization (prl) mutations (7, 8, 57). We refer to the L. monocytogenes secY (G408R) allele as prlA1 in the remainder of the manuscript.
As in other bacteria, secY is essential in L. monocytogenes (58) and is transcribed with other essential genes in a 28-gene operon. Attempts to introduce the prlA1 mutation into a WT background by allelic exchange at the native secY locus were unsuccessful. Therefore, we used phage transduction utilizing a Himar1 transposon insertion in a neighboring gene (lmo2637) as an antibiotic marker to transduce the prlA1 allele from the R57 revertant and the WT secY into various strains. Disruption of lmo2637 had a minor effect on swarming motility (see Fig. S3B in the supplemental material) but no effect on plaque formation (see Fig. S3C in the supplemental material) or in vivo virulence (see Fig. S2C and D in the supplemental material).
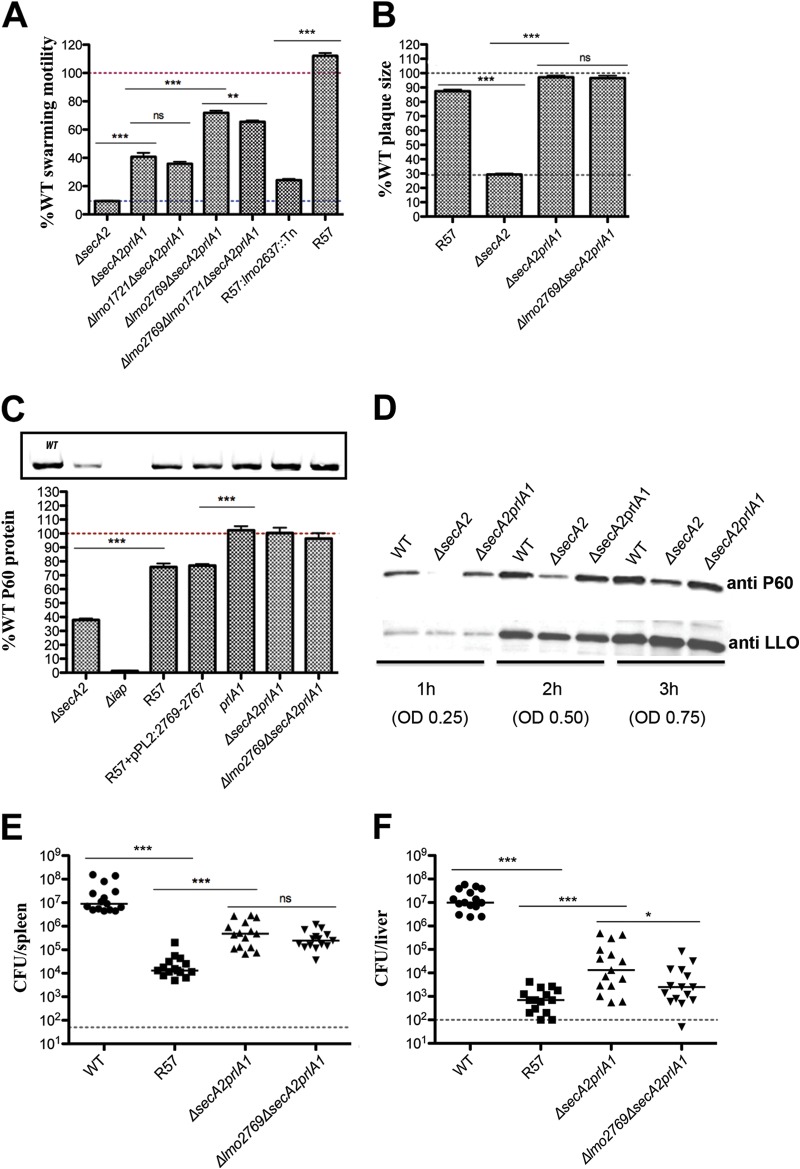
Introduction of the prlA1 mutation into the ΔsecA2 strain or the ΔsecA2 Δlmo2769 double mutant resulted in enhanced motility (Fig. 5A) and restored the smooth colony morphology of these mutants. In addition, motility of R57 diminished significantly (88%) upon replacement of prlA1 with the WT allele (R57:lmo2637::Tn strain) (Fig. 5A), which was coupled with a change in colony morphology from smooth to rough. This suggested that the prlA1 mutation significantly contributed to the R57 motility and cell morphology phenotypes.
FIG 5.
Characterization of the prlA1 mutation. (A) Swarming motility of mutants and strains complemented with the lmo2767 operon was assessed on semisolid LB agar following incubation at 30°C after 48 h. Motility is expressed as a percentage of the swarming area of the WT strain and set at 100% (represented by a red dotted line). Error bars represent standard deviations of the mean zone ratios from three independent experiments. (B) Plaque area of each mutant is shown as a percentage compared to that of the WT strain, set at 100% (represented by a dotted line). Error bars represent standard deviations of the mean plaque size ratios from three independent experiments. Student's t test was used to analyze statistical significance: ***, P < 0.0001; **, P < 0.01; *, P < 0.05; and ns, P ≥ 0.05. (C) Secreted levels of P60 in supernatants from mid-log (5-h) cultured cells in LB broth expressed as a percentage of protein secreted by the WT strain. (D) Secreted levels of P60 and LLO in supernatants from 1-h, 2-h, and 3-h cultures in LB broth. Bacterial burdens in spleens (E) and livers (F) 48 h post-intravenous infection of 1 × 105 CFU in mice, showing data from three independent experiments. The dashed line represents the limit of detection. Statistical significance was evaluated using a Mann-Whitney test. ***, P < 0.0001; *, P < 0.05; ns, P ≥ 0.05.
Surprisingly the prlA1 mutation alone was sufficient to completely restore cell-to-cell spread of the ΔsecA2 strain and the Δlmo2769 ΔsecA2 double mutant (Fig. 5B), while no change was observed for prlA1 alone in a WT background (see Fig. S3C in the supplemental material). Exchanging the prlA1 mutation for the WT allele in R57 (R57:lmo2637::Tn strain) decreased plaque formation from 87% to 39%, the level observed for the Δlmo2769 ΔsecA2 double mutant (see Fig. S3C in the supplemental material).
To investigate whether destabilization of the SecY channel in response to the prlA1 mutation was sufficient to restore protein secretion in ΔsecA2 mutants, the abundance of P60 in the supernatants of mutant cultures was evaluated as a proxy for other SecA2-dependent proteins. Notably, P60 levels were completely restored to WT levels in the secA2 constructs expressing the prlA1 mutation, while the prlA1 strain maintained normal levels of the secreted protein (Fig. 5C). Additionally, the intracellular accumulation of P60 observed in the ΔsecA2 strain (14) was diminished in the ΔsecA2prlA1 strain (data not shown). Restoration of P60 was entirely PrlA1 dependent, as exchanging prlA1 for the WT allele in the R57 background (R57:lmo2637::Tn strain) diminished the secreted P60 levels to those observed for the ΔsecA2 parent strain (see Fig. S3D in the supplemental material). Further, to address the possibility of differing secretion levels between the WT and ΔsecA2prlA1 strains, we undertook a timed experiment in which secretion levels of P60 were assessed. The introduction of the prlA1 mutation restored the levels of P60 secretion in the ΔsecA2 strain, while not affecting the secretion of SecA-dependent listeriolysin O (LLO) (Fig. 5D). Interestingly, P60 levels of the R57 mutant were only 80% of those of the WT strain (Fig. 5C) and were not directly linked to the Δlmo1721 (see Fig. S3E in the supplemental material) or Δlmo2769 (Fig. 5C) mutation. Collectively these observations show that a prlA1 mutation alone was sufficient to overcome the motility, cell morphology, and cell-to-cell spread phenotypes associated with the ΔsecA2 strain, by restoring secretion of the autolysin P60 and likely other SecA2-depended proteins to WT levels.
Lastly, the effect of the prlA1 allele on virulence in vivo was assessed. Somewhat surprisingly, a prlA1 mutation alone in a WT background did not affect virulence (see Fig. S2C and D in the supplemental material). However, virulence of both the ΔsecA2 strain and the Δlmo2769 ΔsecA2 double mutant was partially restored (Fig. 5E and F; see also Fig. S2E and F in the supplemental material). Whereas the secA2 mutant and the R57 suppressor, compared to the WT, were 1,000-fold less virulent in the spleen and 10,000-fold less virulent in the liver (Fig. 3C and D and Fig. 5E and F), the prlA1 mutation partially rescued virulence by approximately 100-fold in the spleen and 20-fold in the liver (Fig. 5E and F). Notably though, it was still considerably less virulent than the WT (20-fold in the spleen and 1,000-fold in the liver).
DISCUSSION
Much of what is known about the general secretory pathway (Sec) in bacteria originates from seminal genetic studies that led to the identification and characterization of the Sec components, including the SecYEG channel and SecA ATPase (3–9). While the secA gene is essential in all bacteria, a subset of Gram-positive bacteria harbor an additional, nonessential homologous gene called secA2 (13–15, 17, 20, 59). Both pathogenic and nonpathogenic species of Listeria encode SecA2 (60), which facilitates the secretion of two major autolysins contributing to septation, swarming motility, plaque formation in tissue culture cells, and virulence in mice (14, 25, 28, 60). It remains unclear, however, why certain bacteria carry secA2 while others do not, and why secA2 is dispensable for cell viability. In this study, we took advantage of the swarming motility defect to isolate spontaneous mutants capable of swarming motility (Fig. 1). One mutant was phenotypically very similar to the WT and was characterized in depth, with its phenotype being genetically traced directly to a single amino acid mutation in SecY. Similar mutations in E. coli secY, called prlA alleles, are gain-of-function mutations that expand the repertoire of substrates that can be exported (6–8, 10, 11). We hypothesize that the secY mutant identified in this study, here named prlA1, rescued the L. monocytogenes secA2 mutant by allowing for secretion of proteins that are normally SecA2 dependent. Recent work in Mycobacterium smegmatis reported that increased SecY levels suppressed the severe phenotypes of a secA2 mutant (21), suggesting that SecA2-dependent protein secretion occurs through interaction with the canonical SecY. Thus, in both L. monocytogenes and M. smegmatis, the canonical secY is very likely part of the translocation pore for SecA2 substrates, in unlike other pathogenic bacteria, including Bacillus anthracis and some Streptococcus and Staphylococcus species, all of which have SecA2 and SecY2 (13, 17, 20, 59).
Wild-type L. monocytogenes is highly lysozyme resistant (45–47). One curious finding reported in this study was that all 70 suppressors were lysozyme sensitive, and in four of the five sequenced suppressor mutants, lysozyme sensitivity was caused by mutations in an uncharacterized ABC transporter encoded by the lmo2769-lmo2767 operon. Lysozyme susceptibility of the fifth mutant was likely due to a mutation in walI, which was previously shown to be required for lysozyme resistance (46). The first gene in the lmo2769-lmo2767 operon encodes an ATP-binding protein similar to a poorly characterized Bacillus subtilis protein (YtrB) whose expression has been observed following exposure to cell wall-acting agents (48). Mutation of either lmo2769 or lmo2768 conferred lysozyme susceptibility, suggesting that these mutations altered the cell wall and may have led to decreased chaining and increased swarming. Although the exact function of this operon requires further characterization, these data suggest that the lmo2769 operon is involved in maintaining cell wall homeostasis (61, 62). The high frequency of mutations detected in this operon, associated with increased swarming, likely provided a growth advantage in semisolid media that increased the probability of acquiring less frequent mutations, such as prlA1.
The prlA1 allele characterized in this study resulted in a glycine substitution for arginine (G408R) in the tenth transmembrane helix of SecY. Variations in this region of SecY in E. coli, such as L407R (PrlA301) and I408N (PrlA4), as well as a PrlA-like mutation in the accessory SecY2 of Streptococcus gordonii (19), promote translocation of proteins with defective signal peptides by destabilizing the pore ring within the SecYEG channel (7, 8, 57). It is likely that the substitution of glycine for a positively charged hydrophilic arginine residue would also induce a conformational change to the pore ring. Further investigation using more direct approaches is required to explain how the prlA1 mutation is able to rescue the SecA2-dependent secretion in L. monocytogenes secA2 mutants. It is possible, however, that the rescue in protein secretion is a result of a more efficient interaction between SecA and SecY induced by the conformational change of the pore ring, similar to that observed in a PrlA4 variant of E. coli (10, 63).
Protein secretion is an energetically costly process, and in the absence of SecA2, another ATPase, such as SecA, is required to provide energy for translocation of P60 in the L. monocytogenes ΔsecA2prlA1 mutant. Since SecA is dimeric during protein translocation (64), it is possible that SecA2 and SecA form a heterodimer which interacts with SecYEG, thereby modulating substrate specificity of the channel. The interaction between SecA2 and the canonical SecA ATPase has previously been shown to occur in Streptococcus (65) and implied in Mycobacterium (66) and more recently in Listeria (67). In the absence of a functional SecA2, SecA may drive SecA2-dependent secretion, although at a much lower rate, potentially due to a lower affinity of SecA alone for the specific substrates. This view is supported by the observation that the export of P60 and other SecA2-dependent proteins is not completely abolished in the secA2 mutant (14, 25).
Although it is not yet characterized, we also identified a suppressor mutation in secA, providing further support for a possible interaction between SecA2 and SecA in L. monocytogenes. This mutation resulted in the D599N amino acid substitution, corresponding to D649 in the second nucleotide-binding domain of SecA in E. coli. Amino acid alterations in this region of SecA, in particular residues 631 to 653, are known as azi, as these confer azide resistance resulting from an increased ATPase activity (68, 69) in addition to having an increased affinity for SecY (69). A SecA homodimer with enhanced affinity for SecY either through prlA1 or azi mutations may suffice in overcoming the requirement for SecA2 in a secA2 mutant. The enhanced interaction of SecA with SecY would indirectly increase the rate of secretion of SecA2-dependent proteins independent of altering the affinity of the ATPase for these substrates.
Another piece of evidence supporting the interaction between the two ATPases comes from a recent proteomics study, which showed that P60 secretion is indistinguishable between WT L. monocytogenes EGD-e and the ΔsecA2 strain when grown in a defined medium at 37°C but was diminished in the mutant at 20°C (32), thus suggesting that SecA2-dependent secretion is able to engage an alternative pathway under different environmental conditions. One ATPase or dimer combination may be favored over the other depending on the energetic state of the cell. For example, the affinity of SecA2 for ATP in Mycobacterium is higher than that of SecA (70). In order not to compete for substrate, SecA2 is bound to ADP in a dormant state until it is required (71). Considering there is no conservation in the signal peptides of proteins being secreted through the SecA2-dependent pathway in L. monocytogenes, it remains unclear how proteins are targeted to SecA2 (14, 32) and how that can be overcome in certain conditions.
It is curious why Listeria species require SecA2 for normal septation, while most other bacteria use other, SecA2-independent, mechanisms. One possibility is that secA2 mutants have an advantage under certain conditions. Indeed, secA2 mutants arise spontaneously in the lab as rough colonies that emanate from a smooth colony and spread on solid media (25–27). At ambient temperatures, these mutants readily form distinct filamentous biofilms, which are thicker than those formed by the WT strain (26, 72). The spontaneous and reversible (25, 73) morphological conversion to a rough phenotype may provide a potential advantage to L. monocytogenes in its saprophytic phase by enhancing the ability to colonize abiotic surfaces. This may also be one of the reasons why the nonpathogenic Listeria species have retained SecA2; however, this potential advantage outside the host comes at a significant cost to its pathogenic phase, as L. monocytogenes secA2 mutants are approximately 1,000-fold attenuated for virulence in mice (Fig. 3C and D) (14, 25, 28), although rough mutants can persist in the gut (73) and the gallbladder (74). Some of this attenuation has been traced to the lower levels of secreted autolysins, primarily P60, and to a lesser extent NamA (14). However, even though the prlA1 mutation described in the study had normal levels of secreted LLO and restored WT levels of secreted P60 and cell-to-cell spread, it resulted in only a 100-fold rescue in virulence in vivo of the attenuated secA2 mutant. The suppressor mutant was still less virulent than the WT strain, suggesting that a functional SecA2 is still required for full expression of L. monocytogenes virulence. SecA2-dependent secretion may have evolved in L. monocytogenes to provide a mechanism for switching between the parasitic and the saprophytic phase, thus providing an advantage in its ability to thrive in the environment as well as inside a host.
Supplementary Material
ACKNOWLEDGMENTS
Daniel A. Portnoy has a consulting relationship with and a financial interest in Aduro Biotech, and both he and the company stand to benefit from the commercialization of the results of this research.
This work was supported by National Institutes of Health grant 1PO1 AI63302 and National Institutes of Health grant 1R01 AI27655 to D.A.P. (http://www.nih.gov). This work used the Vincent J. Coates Genomics Sequencing Laboratory at UC Berkeley, supported by NIH S10 Instrumentation grants S10RR029668 and S10RR027303.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02284-14.
REFERENCES
- 1.du Plessis DJF, Nouwen N, Driessen AJM. 2011. The Sec translocase. Biochim Biophys Acta 1808:851–865. doi: 10.1016/j.bbamem.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Lycklama a Nijeholt JA, Driessen AJM. 2012. The bacterial Sec-translocase: structure and mechanism. Philos Trans R Soc B 367:1016–1028. doi: 10.1098/rstb.2011.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckwith J. 2013. The Sec-dependent pathway. Res Microbiol 164:497–504. doi: 10.1016/j.resmic.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusters I, Driessen AJM. 2011. SecA, a remarkable nanomachine. Cell Mol Life Sci 68:2053–2066. doi: 10.1007/s00018-011-0681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam PCK, Maillard AP, Chan KKY, Duong F. 2005. Investigating the SecY plug movement at the SecYEG translocation channel. EMBO J 24:3380–3388. doi: 10.1038/sj.emboj.7600804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flower AM. 2007. The SecY translocation complex: convergence of genetics and structure. Trends Microbiol 15:203–210. doi: 10.1016/j.tim.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Osborne RS, Silhavy TJ. 1993. PrlA suppressor mutations cluster in regions corresponding to three distinct topological domains. EMBO J 12:3391–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MA, Clemons WM, DeMars CJ, Flower AM. 2005. Modeling the effects of prl mutations on the Escherichia coli SecY complex. J Bacteriol 187:6454–6465. doi: 10.1128/JB.187.18.6454-6465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duong F, Wickner W. 1999. The PrlA and PrlG phenotypes are caused by a loosened association among the translocase SecYEG subunits. EMBO J 18:3263–3270. doi: 10.1093/emboj/18.12.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Wolk JPW, Kekkes P, Boorsma A, Huie JL, Silhavy TJ, Driessen AJM. 1998. PrlA4 prevents the rejection of signal sequence defective preproteins by stabilizing the SecA-SecY interaction during the initiation of translocation. EMBO J 17:3631–3639. doi: 10.1093/emboj/17.13.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emr SD, Hanley-Way S, Silhavy TJ. 1981. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell 23:79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- 12.Bieker KL, Phillips GJ, Silhavy TJ. 1990. The sec and prl genes of Escherichia coli. J Bioenerg Biomembr 22:291–310. doi: 10.1007/BF00763169. [DOI] [PubMed] [Google Scholar]
- 13.Feltcher ME, Braunstein M. 2012. Emerging themes in SecA2-mediated protein export. Nat Rev Microbiol 10:779–789. doi: 10.1038/nrmicro2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenz LL, Mohammadi S, Geissler A, Portnoy DA. 2003. SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis. Proc Natl Acad Sci U S A 100:12432–12437. doi: 10.1073/pnas.2133653100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigel NW, Braunstein M. 2008. A new twist on an old pathway—accessory secretion systems. Mol Microbiol 69:291–302. doi: 10.1111/j.1365-2958.2008.06294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feltcher ME, Gibbons HS, Ligon LS, Braunstein M. 2013. Protein export by the mycobacterial SecA2 system is determined by the preprotein mature domain. J Bacteriol 195:672–681. doi: 10.1128/JB.02032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bensing BA, Seepersaud R, Yen YT, Sullam PM. 2014. Selective transport by SecA2: an expanding family of customized motor proteins. Biochim Biophys Acta 1843:1674–1686. doi: 10.1016/j.bbamcr.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bensing BA, Sullam PM. 2009. Characterization of Streptococcus gordonii SecA2 as a paralogue of SecA. J Bacteriol 191:3482–3491. doi: 10.1128/JB.00365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bensing BA, Sullam PM. 2010. Transport of preproteins by the accessory Sec system requires a specific domain adjacent to the signal peptide. J Bacteriol 192:4223–4232. doi: 10.1128/JB.00373-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneewind O, Missiakas DM. 2012. Protein secretion and surface display in Gram-positive bacteria. Philos Trans R Soc B 367:1123–1139. doi: 10.1098/rstb.2011.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ligon LS, Rigel NW, Romanchuk A, Jones CD, Braunstein M. 2013. Suppressor analysis reveals a role for SecY in the SecA2-dependent protein export pathway of mycobacteria. J Bacteriol 195:4456–4465. doi: 10.1128/JB.00630-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan JT, Young EF, McCann JR, Braunstein M. 2012. The Mycobacterium tuberculosis SecA2 system subverts phagosome maturation to promote growth in macrophages. Infect Immun 80:996–1006. doi: 10.1128/IAI.05987-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. 2001. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev 14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlech WF., III 2000. Foodborne listeriosis. Clin Infect Dis 31:770–775. doi: 10.1086/314008. [DOI] [PubMed] [Google Scholar]
- 25.Lenz LL, Portnoy DA. 2002. Identification of a second Listeria secA gene associated with protein secretion and the rough phenotype. Mol Microbiol 45:1043–1056. doi: 10.1046/j.1365-2958.2002.03072.x. [DOI] [PubMed] [Google Scholar]
- 26.Monk IR, Cook GM, Monk BC, Bremer PJ. 2004. Morphotypic conversion in Listeria monocytogenes biofilm formation: biological significance of rough colony isolates. Appl Environ Microbiol 70:6686–6694. doi: 10.1128/AEM.70.11.6686-6694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutekunst KA, Pine L, White E, Kathariou S, Carlone GM. 1992. A filamentous-like mutant of Listeria monocytogenes with reduced expression of a 60-kilodalton extracellular protein invades and grows in 3T6 and Caco-2 cells. Can J Microbiol 38:843–851. doi: 10.1139/m92-137. [DOI] [PubMed] [Google Scholar]
- 28.Machata S, Hain T, Rohde M, Chakraborty T. 2005. Simultaneous deficiency of both MurA and p60 proteins generates a rough phenotype in Listeria monocytogenes. J Bacteriol 187:8385–8394. doi: 10.1128/JB.187.24.8385-8394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Archambaud C, Nahori MA, Pizarro-Cerda J, Cossart P, Dussurget O. 2006. Control of Listeria superoxide dismutase by phosphorylation. J Biol Chem 281:31812–31822. doi: 10.1074/jbc.M606249200. [DOI] [PubMed] [Google Scholar]
- 30.Burkholder KM, Kim K, Mishra KK, Medina S, Hahm B, Kim H, Bhunia AK. 2009. Expression of LAP, a SecA2-dependent secretory protein, is induced under anaerobic environment. Microbes Infect 11:859–867. doi: 10.1016/j.micinf.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Port GC, Freitag NE. 2007. Identification of novel Listeria monocytogenes secreted virulence factors following mutational activation of the central virulence regulator, PrfA. Infect Immun 75:5886–5897. doi: 10.1128/IAI.00845-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renier S, Chambon C, Viala D, Chagnot C, Hébraud M, Desvaux M. 2013. Exoproteomic analysis of the SecA2-dependent secretion in Listeria monocytogenes EGD-e. J Proteomics 80:183–195. doi: 10.1016/j.jprot.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 33.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 34.Sun AN, Camilli A, Portnoy DA. 1990. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun 58:3770–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup . 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camilli A, Tilney LG, Portnoy DA. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol 8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauer P, Chow MYN, Loessner MJ, Portnoy DA, Calendar R. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J Bacteriol 184:4177–4186. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodgson DA. 2000. Generalized transduction of serotype 1/2 and serotype 4b strains of Listeria monocytogenes. Mol Microbiol 35:312–323. doi: 10.1046/j.1365-2958.2000.01643.x. [DOI] [PubMed] [Google Scholar]
- 40.Witte CE, Whiteley AT, Burke TP, Sauer JD, Portnoy DA, Woodward JJ. 2013. Cyclic di-AMP is critical for Listeria monocytogenes growth, cell wall homeostasis, and establishment of infection. mBio 4(3):e00282–13. doi: 10.1128/mBio.00282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zemansky J, Kline BC, Woodward JJ, Leber JH, Marquis H, Portnoy DA. 2009. Development of a mariner-based transposon and identification of Listeria monocytogenes determinants, including the peptidyl-prolyl isomerase PrsA2, that contribute to its hemolytic phenotype. J Bacteriol 191:3950–3964. doi: 10.1128/JB.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blackman SA, Smith TJ, Foster SJ. 1998. The role of autolysins during vegetative growth of Bacillus subtilis 168. Microbiology 144:73–82. [DOI] [PubMed] [Google Scholar]
- 43.Halbedel S, Hahn B, Daniel RA, Flieger A. 2012. DivIVA affects secretion of virulence-related autolysins in Listeria monocytogenes. Mol Microbiol 83:821–839. doi: 10.1111/j.1365-2958.2012.07969.x. [DOI] [PubMed] [Google Scholar]
- 44.Rashid MH, Kuroda A, Sekiguchi J. 1993. Bacillus subtilis mutant deficient in the major autolytic amidase and glucosaminidase is impaired in motility. FEMS Microbiol Lett 112:135–140. doi: 10.1111/j.1574-6968.1993.tb06438.x. [DOI] [PubMed] [Google Scholar]
- 45.Rae CS, Geissler A, Adamson PC, Portnoy DA. 2011. Mutations of the Listeria monocytogenes peptidoglycan N-deacetylase and O-acetylase result in enhanced lysozyme sensitivity, bacteriolysis, and hyperinduction of innate immune pathways. Infect Immun 79:3596–3606. doi: 10.1128/IAI.00077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burke TP, Loukitcheva A, Zemansky J, Wheeler R, Boneca IG, Portnoy DA. 2014. Listeria monocytogenes is resistant to lysozyme by the regulation, not acquisition, of cell wall modifying enzymes. J Bacteriol 196:3756–3767. doi: 10.1128/JB.02053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boneca IG, Dussurget O, Cabanes D, Nahori M, Sousa S, Lecuit M, Psylinakis E, Bouriotis V, Hugot J, Giovannini M, Coyle A, Bertin J, Namane A, Rousselle J, Cayet N, Prévost M, Balloy V, Chignard M, Philpott DJ, Cossart P, Girardin SE. 2007. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc Natl Acad Sci U S A 104:997–1002. doi: 10.1073/pnas.0609672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roche SM, Velge P, Bottreau E, Durier C, Marquet-van der Mee N, Pardon P. 2001. Assessment of the virulence of Listeria monocytogenes: agreement between a plaque-forming assay with HT-29 cells and infection of immunocompetent mice. Int J Food Microbiol 68:33–44. doi: 10.1016/S0168-1605(01)00460-3. [DOI] [PubMed] [Google Scholar]
- 49.Mérino D, Réglier-Poupet H, Berche P, Charbit A, European Listeria Genome Consortium . 2002. A hypermutator phenotype attenuates the virulence of Listeria monocytogenes in a mouse model. Mol Microbiol 44:877–887. doi: 10.1046/j.1365-2958.2002.02929.x. [DOI] [PubMed] [Google Scholar]
- 50.Dyer CM, Vartanian AS, Zhou H, Dahlquist FW. 2009. A molecular mechanism of bacterial flagellar motor switching. J Mol Biol 388:71–84. doi: 10.1016/j.jmb.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kihara M, Miller GU, Macnab RM. 2000. Deletion analysis of the flagellar switch protein FliG of Salmonella. J Bacteriol 182:3022–3028. doi: 10.1128/JB.182.11.3022-3028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mariconda S, Wang Q, Harshey RM. 2006. A mechanical role for the chemotaxis system in swarming motility. Mol Microbiol 60:1590–1602. doi: 10.1111/j.1365-2958.2006.05208.x. [DOI] [PubMed] [Google Scholar]
- 53.Toker AS, Kihara M, Macnab RM. 1996. Deletion analysis of the FliM flagellar switch protein of Salmonella Typhimurium. J Bacteriol 178:7069–7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milenbachs Lukowiak A, Mueller KJ, Freitag NE, Youngman P. 2004. Deregulation of Listeria monocytogenes virulence gene expression by two distinct and semi-independent pathways. Microbiology 150:321–333. doi: 10.1099/mic.0.26718-0. [DOI] [PubMed] [Google Scholar]
- 55.Stoll R, Goebel W. 2010. The major PEP-phosphotransferase systems (PTSs) for glucose, mannose and cellobiose of Listeria monocytogenes, and their significance for extra- and intracellular growth. Microbiology 156:1069–1083. doi: 10.1099/mic.0.034934-0. [DOI] [PubMed] [Google Scholar]
- 56.Dalet K, Arous S, Cenatiempo Y, Héchard Y. 2003. Characterization of a unique σ54-dependent PTS operon of the lactose family in Listeria monocytogenes. Biochimie 85:633–638. doi: 10.1016/S0300-9084(03)00134-2. [DOI] [PubMed] [Google Scholar]
- 57.Lycklama a Nijeholt JA, Bulacu M, Marrink SJ, Driessen AJM. 2010. Immobilization of the plug domain inside the SecY channel allows unrestricted protein translocation. J Biol Chem 285:23747–23754. doi: 10.1074/jbc.M110.124636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hossain MM, Mosnaz ATMJ, Sajib AM, Roy PK, Shakil SK, Ullah SMS, Prodhan SH. 2013. Identification of putative drug targets of Listeria monocytogenes F2365 by subtractive genomics approach. J Bio Sci Biotech 2:63–71. [Google Scholar]
- 59.Nguyen-Mau S, Oh S, Kern VJ, Missiakas DM, Schneewind O. 2012. Secretion genes as determinants of Bacillus anthracis chain length. J Bacteriol 194:3841–3850. doi: 10.1128/JB.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mishra KK, Mendonca M, Aroonnual A, Burkholder KM, Bhunia AK. 2011. Genetic organisation and molecular characterization of secA2 locus in Listeria species. Gene 489:76–85. doi: 10.1016/j.gene.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 61.Collins B, Curtis N, Cotter PD, Hill C, Ross RP. 2010. The ABC transporter AnrAB contributes to the innate resistance of Listeria monocytogenes to nisin, bacitracin, and various β-lactam antibiotics. Antimicrob Agents Chemother 54:4416–4423. doi: 10.1128/AAC.00503-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cuthbertson L, Kos V, Whitfield C. 2010. ABC transporters involved in export of cell surface glycoconjugates. Microbiol Mol Biol Rev 74:341–362. doi: 10.1128/MMBR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Keyzer J, van der Does C, Swaving J, Driessen AJM. 2002. The F286Y mutation of PrlA4 tempers the signal sequence suppressor phenotype by reducing the SecA binding affinity. FEBS Lett 510:17–21. doi: 10.1016/S0014-5793(01)03213-6. [DOI] [PubMed] [Google Scholar]
- 64.Kusters I, van den Bogaart G, Kedrov A, Krasnikov V, Fulyani F, Poolman B, Driessen AJM. 2011. Quaternary structure of SecA in solution and bound to SecYEG probed at the single molecule level. Structure 19:430–439. doi: 10.1016/j.str.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 65.Zhou M, Zhang H, Zhu F, Wu H. 2011. Canonical SecA associates with an accessory secretory protein complex involved in biogenesis of a streptococcal serine-rich repeat glycoprotein. J Bacteriol 193:6560–6566. doi: 10.1128/JB.05668-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rigel NW, Gibbons HS, McCann JR, McDonough JA, Kurtz S, Braunstein M. 2009. The accessory SecA2 system of mycobacteria requires ATP binding and the canonical SecA1. J Biol Chem 284:9927–9936. doi: 10.1074/jbc.M900325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halbedel S, Reiss S, Hahn B, Albrecht D, Mannala GK, Chakraborty T, Hain T, Engelmann S, Flieger A. 2014. A systematic proteomic analysis of Listeria monocytogenes house-keeping protein secretion systems. Mol Cell Proteomics 13:3063–3081. doi: 10.1074/mcp.M114.041327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Das S, Grady LM, Michtavy J, Zhou Y, Cohan FM, Hingorani MM. 2012. The variable subdomain of Escherichia coli SecA functions to regulate SecA ATPase activity and ADP release. J Bacteriol 194:2205–2213. doi: 10.1128/JB.00039-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmidt M, Ding H, Ramamurthy V, Mukerji I, Oviler D. 2000. Nucleotide binding activity of SecA homodimer is conformationally regulated by temperature and altered by prlD and azi mutations. J Biol Chem 275:15440–15448. doi: 10.1074/jbc.M000605200. [DOI] [PubMed] [Google Scholar]
- 70.Hou JM, D'Lima NG, Rigel NW, Gibbons HS, McCann JR, Braunstein M, Teschke CM. 2008. ATPase activity of Mycobacterium tuberculosis SecA1 and SecA2 proteins and its importance for SecA2 function in macrophages. J Bacteriol 190:4880–4887. doi: 10.1128/JB.00412-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D'Lima NG, Teschke CM. 2014. ADP-dependent conformational changes distinguish Mycobacterium tuberculosis SecA2 from SecA1. J Biol Chem 289:2307–2317. doi: 10.1074/jbc.M113.533323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Renier S, Chagnot C, Deschamps J, Caccia N, Szlavik J, Joyce SA, Popowska M, Hill C, Knøchel S, Briandet R, Hébraud M, Desvaux M. 2014. Inactivation of the SecA2 protein export pathway in Listeria monocytogenes promotes cell aggregation, impacts biofilm architecture and induces biofilm formation in environmental condition. Environ Microbiol 16:1176–1192. doi: 10.1111/1462-2920.12257. [DOI] [PubMed] [Google Scholar]
- 73.Zachar Z, Savage DC. 1979. Microbial interference and colonization of the murine gastrointestinal tract by Listeria monocytogenes. Infect Immun 23:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hardy J, Francis KP, DeBoer M, Chu P, Gibbs K, Contag CH. 2004. Extracellular replication of Listeria monocytogenes in the murine gall bladder. Science 303:851–853. doi: 10.1126/science.1092712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.