Summary
Brain metastasis is an end stage in breast cancer progression. Traditional treatment options have minimal efficacy, and overall survival is on the order of months. The incidence of brain metastatic disease is increasing with the improved management of systemic disease and prolongation of survival. Unfortunately, the targeted therapies that control systemic disease have diminished efficacy against brain lesions. There are reasons to be optimistic, however, as emerging therapies have shown promise in preclinical and early clinical settings. This review discusses recent advances in breast cancer brain metastasis therapy and potential approaches for successful treatment.
Keywords: brain microenvironment, targeted therapy
Breast cancer brain metastases are an increasing health care problem
The incidence of breast cancer brain metastases (BCBM) varies with the subtype of disease. Whereas patients with estrogen receptor (ER) positive, human epidermal growth factor receptor-2 (HER-2) negative tumors (70% of breast cancers) have a brain metastasis incidence of 5–10%, those with triple-negative or HER2-positive tumors have an incidence rate about 20% and 25–50%, respectively (Kennecke et al., 2010; Aversa et al. 2014). The high incidence rate for patients with HER2-positive disease is likely due to several factors, including the ability of HER2 to increase the proclivity of brain metastases (BM), but is almost certainly due to the prolongation of survival resulting from anti-HER2 targeted therapies (Brufsky et al., 2011b; Gori et al., 2007; Olson et al., 2013). As therapies for systemic disease improve, incidence rates of BCBM are likely to rise. In the majority of cases, treatment is palliative and mostly local, such as surgical resection, stereotactic radiosurgery and/or whole brain irradiation (WBRT) (Eichler et al, 2011). Prognosis is affected by various factors, including the number of BM, the presence of active extracranial disease, the patient’s age and performance status, and the tumor subtype (Sperduto et al., 2013; Sperduto et al., 2012). These factors also affect treatment regimens. Survival duration varies between 4–6 months with WBRT to about 18 months with multimodal therapies (Kocher et al., 2011). The poor prognosis with local therapies, and the fact that most patients with BCBM display synchronous extracranial disease, underscores the need for better systemic treatments with efficacy in the cerebral microenvironment. Over the last few years, preclinical and clinical progress in the treatment of BCBM has led to novel hypotheses for improving therapeutic outcome. This review focuses on these discoveries, separates those confirmed in patients from those still pre-clinical, distinguishes between preventative and treatment strategies, and suggests avenues for future investigation.
Can breast cancer brain metastases be prevented?
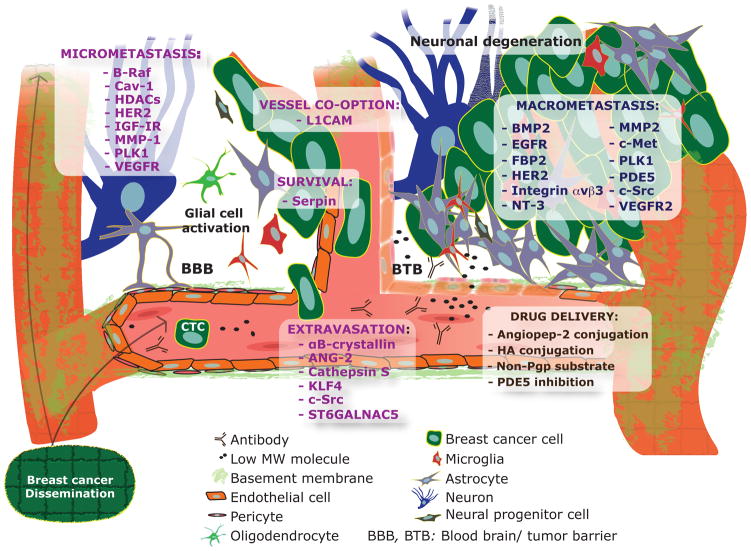
Animal models of BM have provided insights into processes of the brain-metastatic cascade: dissemination of metastasis-competent cells from the primary tumor, intravasation into the blood circulation, active or passive migration towards the target organ, embedding into a capillary bed and attachment to the endothelium, extravasation through the blood-brain barrier (BBB), and expansion in the brain microenvironment (Figure 1) (Eichler et al., 2011). Once arrested within the capillary bed of the brain circulation, metastatic cancer cells come in contact with brain microvascular endothelial cells, which promote cancer cell growth and invasion. Real-time imaging of a murine brain metastasis model showed early extravasation and persistent contact with microvessels as necessary elements for colonization (Kienast et al., 2010). A separate study identified the cell adhesion molecule L1 (L1CAM) as necessary for vascular co-option and, therefore, metastatic cancer cell survival and tumor initiation in the brain microenvironment (Valiente et al., 2014).
Figure 1. Schematic of notable targets of BCBM formation identified in preclinical studies (see Table 1).
Molecules are categorized based on the stage of the metastatic cascade in which it is involved. Brain-tropic circulating tumors cells (CTC) may express a particular signature, such as EpCAM-/HER2+/EGFR+/HPSE+/Notch1+ (Zhang et al., 2013). While drug delivery into brain metastatic lesions is compromised by the BTB, the ease of access is greater than in the normal brain (with an intact BBB). Methods used to enhance drug delivery are also mentioned.
The majority of preclinical studies focus on early stages of BCBM. This is mainly due to the fact that the knowledge gained from preclinical studies is limited to the models and treatment methods employed. While models of spontaneous brain metastasis from intra-mammary implanted breast cancer cells exist, the majority of knowledge has been gained from intracardiac, intracarotid, or intracranial injection models that forgo invasion and migratory escape from the primary tumor environs. Multiple selection rounds of brain metastatic lesions after mammary fat pad, intracarotid, or intracardiac injection have generated “brain-seeking” clonal sublines. Gene expression analysis between brain-seeking and parental lines identified genes involved in the early stages of the BCBM cascade. In addition, the majority of studies involve treatments initiated before the establishment of BCBM. Analysis of the initial steps of brain metastatic colonization revealed that intravascularly injected cancer cells colonize the brain beginning at day 7–10 post injection (Kienast et al., 2010; Lorger and Felding-Habermann, 2010). Treatment studies that begin prior to colonization translate, clinically, into prevention studies. Table 1 summarizes salient findings from preclinical prevention studies for each of the specific process of the brain metastatic cascade (Figure 1). If preventative measures are to succeed in the clinic, methods to identify predisposed patients are necessary, and this will entail identification of the expression of relevant proteins in primary or systemic metastases of patient tissue (Table 1). In addition to biopsy or resected tissue, circulating tumor cells (CTCs) have important prognostic and therapeutic implications in the prevention setting. Zhang, et al. (Zhang et al., 2013) identified a potential signature of BCBM in human CTCs that has the potential to identify patients susceptible to brain metastatic disease.
Table 1. Preclinical studies of BCBM.
Studies are arranged chronologically. The target(s) for each study is mentioned as well as the method of inhibiting/activating the target. Targeting methods that represent therapies presently suitable for clinical translation are italicized. The preclinical model used as well as outcome observed is included. Unless stated otherwise, the studies used human derived breast cancer cell lines implanted into immunodeficient mice. Studies with the potential for translation into treatment trials in the clinic are noted in bold in the comments section. Studies addressing enhanced drug delivery are also noted in the comments section.
| Target | Targeting Method | Cell lines/Delivery | Outcome | Comments | References |
|---|---|---|---|---|---|
| Human epidermal growth factor receptor 2 (HER2) | Trastuzumab | MCF-7-HER2 / Intracranial injection of athymic rat | Increased survival | Direct intracerebral microinfusion. | Grossi PM et al. 2003 |
| Vascular Endothelial Growth Factor Receptor (VEGFR) | PTK787/Z 222584 (RTK inhibitor) | MDA-231-BR3 / Intracarotid | Decreased BM / No survival benefit | Brain-seeking clones express more VEGF-A. | Kim LS et al. 2004 |
| Matrix-metalloproteinase 2 (MMP-2) | TIMP2 | ENU1564 rat mammary adenocarcinoma cells / Intraventricular | Decreased BM | TIMP2 expression decreases primary tumor growth as well. | Mendes O et al. 2007 |
| Immunostimulatory CpG | CpG oligodeoxynucleotides (ODN) | EMT6 murine mammary carcinoma cells / Intracranial injection of CpG-ODN challenged mice | Decreased BM | Induction of protective immunity in the brain. No beneficial effect on established BM | Xiong Z et al. 2008 |
| Histone deacetylase (HDAC) | Vorinostat | MDA-MB-231-BR / Intracranial | Decreased BM / Increased survival | Combination of Vorinostat with radiotherapy conveys better survival and further decreased BM when compared to monotherapy. Treatment study | Baschnagel A et al. 2009 |
| ST6GALNAC5 (Sialyltransferase) | shRNA | MDA-MB-231-BR, CN34-BrM2c / Intracarotid | Decreased BM / Increased survival | ST6GALNAC5 expression enhances adhesion to brain endothelial cells and promotes passage through the BBB. Addition of cetuximab (anti-EGFR antibody) further reduces BM. Increased expression in BM clinical tissue. | Bos PD et al. 2009 |
| Integrin αvβ3 | Plasmid-mediated expression of αvβ3 mutant (non-activated) | MDA-MB-435 / Intracranial | Decreased BM | Tumor cell integrin αvβ3 activation increases angiogenesis and decreases hypoxia. | Lorger M et al. 2009 |
| HDAC | Vorinostat | MDA-MB-231-BR / Intracardiac | Decreased BM | No significant decrease in BM observed when treatment is delayed to 18 days post-injection | Palmieri D et al. 2009 |
| Phosphodiesterase 5 (PDE5) / HER2 | Vardenafil (PDE5 inhibitor) / trastuzumab | BT-474 / Intracranial | Increased survival | PDE5 inhibition increases trastuzumab delivery in brain. Treatment study | Hu J et al. 2010 |
| Caveolin-1 and Signal transducer and activator of transcription 3 (Stat3) | Suppressor Of Cytokine Signaling 1 (SOCS1) expression | MDA-MB-231-BR / Intracarotid | Decreased BM | SOCS-1 regulates Stat3 expression. Stat3 regulates Caveolin-1 expression. Increased pStat3 and decreased caveolin-1 expression in BM clinical tissue. | Chiu WT et al. 2011 |
| Proto-oncogene B-Raf | Pazopanib | MDA-MB-231-BR-HER2 and MCF7- HER2-BR3 / Intracardiac | Decreased BM | Pazopanib does not alter vasculature. | Gril B et al. 2011 |
| Notch1 | shRNA or DAPT (gamma secretase inhibitor) | CD44hi_CD24lo_MDA-MB-231-BR / Intracardiac | Decreased BM | DAPT effective in treating established BM lesions (14 days post injection). | McGowan PM et al. 2011 |
| Polo-like kinase 1 (Plk1) | GSK461364A | MDA-MB-231-BR/ Intracardiac injection | Decreased BM / Increased survival | GSK461364A sensitizes cells to radiation. Delayed delivery of GSK461364A (13 days) also promotes survival. Increased expression in BM clinical tissue. Treatment study | Qian Y et al. 2011 |
| Heparinase | MicroRNA-1258 | MDA-MB-231-BR3 / Intracardiac | Decreased BM | Effect of miR-1258 partly rescued by Heparinase overexpression. Increased Heparinase and decreased miRNA- 1258 expression in BM clinical tissue. | Zhang L et al. 2011 |
| Microtubules | TPI-287 | MDA-MB-231-BR / Intracardiac | Decreased BM | No significant decrease in BM observed when treatment delayed to 18 days post-injection | Fitzgerald DP et al. 2012 |
| Pigment epithelium-derived factor (PEDF) | Plasmid-mediated expression. | MDA-MB-231-BR or murine 4T1-BR / Intracranial or intracardiac | Decreased BM | PEDF previously identified in gene array with human BM tissue. PEDF promotes neuronal survival around BM lesion. PDEF is downregulated in BM clinical tissue. | Fitzgerald DP et al. 2012 |
| HER2 and VEGFR2 | Anti-VEGFR2 (DC101), lapatinib and trastuzumab | BT-474 / Intracranial | Decreased BM / Increased survival | First targeted therapy combination. Treatment study | Kodack D et al. 2012 |
| Met Proto-oncogene (c-Met) | shRNA | MDA-MB-435 / Intracranial | Decreased BM | Survival benefit in intra-internal carotid artery injection model. Increased expression in BM clinical tissue. | Lee SJ et al. 2012 |
| MMP-1 | shRNA | MDA-MB-231-BR and -BR3 / Intracardiac | Decreased BM | Also effective in lung metastasis model. | Liu H et al. 2012 |
| Neurotrophin-3 (NT-3) | shRNA | MDA-MB-361, BCM2 BRainG2 / Intracranial | Decreased BM | NT3 expression decreases microglia activation and increases HER2 expression. Increased expression in BM clinical tissue. | Louie E et al. 2012 |
| Phosphatidylinositide 3-kinase (PI3K) | BKM-120 | Rag2−/−;Il2rg−/− mice / MDA-MB-453, BT-474 / Intravenous and intramammary | Decreased BM | Spontaneous brain metastasis model. Model further recapitulates multi-organ metastasis. | Nanni P et al. 2012 |
| αB-crystallin | shRNA | GILM2 and MDA-MB-231 / Intramammary | Decreased BM | Spontaneous brain metastasis model. αB-crystallin knockdown does not reduce primary tumor growth. Increased expression in BM clinical tissue. | Malin D et al. 2013 |
| Chemotherapy | HA–paclitaxel nanoconjugate | MDA-MB-231-BR / Intracardiac | Increased survival | HA conjugate increases delivery of Paclitaxel into the brain by bypassing p-glycoprotein mediated efflux. | Mittapalli RK et al. 2013 |
| Epidermal growth factor receptor (EGFR) / HER2 | TAK-285 (dual inhibitor) | BT-474 / Intracranial | Decreased BM | Evades efflux mechanism since not a p-glycoprotein substrate. Treatment study | Nakayama A et al. 2013 |
| Bone morphogenetic protein 2 (BMP-2) | shRNA | MDA-MB-231BR / Intracranial | Decreased BM | BMP-2 promotes differentiation of NPCs into astrocytes. Expressed in BM clinical tissue. | Neman J et al. 2013 |
| Kruppel-like factor 4 (KLF4) | miR-7 (regulates expression of the stem cell protein KLF4) | CD24−_CD44+_ESA+ CTCs from 231BrM / Intracardiac | Decreased BM / Increased survival | High KLF4 expression is inversely correlated with brain metastasis-free survival. miR-7 is downregulated and KLF4 upregulated in BM clinical tissue. | Okuda H et al. 2013 |
| Insulin-like growth factor 1 receptor (IGF-1R) | shRNA | MDA-MB-231BR / Intracarotid | Increased survival | Picropodophyllin used to block IGF-IR in vitro but not in vivo. | Saldana SM et al. 2013 |
| Proto-oncogene tyrosine kinase Src (c-Src) | Saracatinib with lapatinib | BT-474-BR and MDA-MB-231-BR / Intracarotid | Decreased BM and Increased survival | Monotherapy does not significantly decrease BM. Effective on established BM. Increased expression in BM clinical tissue. Treatment study | Zhang S et al. 2013 |
| Angiopoietin-2 (Ang-2) | Trebananib | 4T1-BRM5 murine mammary carcinoma cells / Mammary fat pad | Decreased BM | Spontaneous brain metastasis. Trebananib improves BBB integrity. Ang-2 is secreted by endothelial cells. | Avraham HK et al. 2014 |
| Fructose-1,6-bisphosphatase isozyme 2 (FBP-2) | shRNA | 4T1 murine mammary carcinoma cells and MDA-MB-31Br3 / Intracranial | Decreased BM / Increased survival | Knockdown of FBP2 does not reduce primary tumor growth. Highlights microenvironment-specific regulation of tumor metabolism. | Chen J et al. 2014 |
| Cathepsin S | shRNA, and VBY-999 | MDA-MB-231-Br-M PyMT-BrM / Intracardiac | Decreased BM | Both stromal and tumor derived Cathepsin blocked for effect. VBY-999 not effective on established brain metastases. Increased expression in BM clinical tissue. | Sevenich L et al. 2014 |
| Serpins and L1 neural cell adhesion molecule (L1CAM) | shRNA | MDA-MB-231-BrM2 / Intracarotid | Decreased BM / Increased survival | SERPINs also mediate survival of brain metastatic lung cancer cell lines. L1CAM is a major vessel co-option molecule. Increased expression in BM clinical tissue. | Valiente M et al. 2014 |
BM, brain metastases; BBB, blood-brain barrier
Studies investigating the biology of established metastatic lesions and its interaction with the microenvironment are beginning to provide important knowledge about brain colonization. Once infiltrated into the brain tissue, breast cancer cells encounter a number of host cell types, including pericytes, reactive glia, neural progenitor cells, neurons, and oligodendrocytes. Although the survival of neurons is reduced by growing BM (Fitzgerald et al., 2012), there are no studies implicating neurons, oligodendrocytes or pericytes in BM formation. Pericytes are present in BM (Lorger and Felding-Habermann, 2010), and play a significant role in the vasculature of primary brain tumors (Armulik et al., 2011; Cheng et al., 2013). More is known about the role of astrocytes and microglia that surround and infiltrate brain metastatic lesions (Fitzgerald et al., 2008; Lorger and Felding-Habermann, 2010). Analysis of human BCBM shows an abundance of activated astrocytes and microglia around and within the lesion (Zhang and Olsson, 1995). The initial survival of brain metastatic cells seems to depend on their ability to evade astrocyte-induced cell death (Valiente et al., 2014). The cells that survive take advantage of the growth-permissive microenvironment. Preclinical studies have begun to unravel the effect of the brain microenvironment on cancer cells, including its ability to reprogram the gene expression patterns of different cancer cell types (Park et al., 2011). Gene signature analysis revealed alterations in pathways such as proliferation, cell death and metabolism in breast cancer cells. Astrocytes, alone, can alter the gene expression of breast or lung cancer cells, and can promote resistance to chemotherapy through activation of the endothelin axis (Kim et al., 2011; Kim et al., 2014). Recent findings demonstrate that tumor cells can increase the density of growth-permissive astrocytes by promoting the differentiation of neural progenitor cells into astrocytes (Neman et al., 2013). Although the outcome of microglia infiltration is less known, microglia activation is inversely correlated with the growth of breast cancer cells in the brain (Louie et al., 2013). In summary, the brain microenvironment clearly offers a unique milieu in which metastatic cancer cells can survive and proliferate. Pathways altered by the microenvironment that mediate therapeutic resistance are beginning to emerge. Furthermore, clinical BM tissue has been shown to contain carcinoma-associated fibroblasts – not resident to the brain – that could play a significant role in colonization and treatment resistance (Duda et al., 2010).
Despite major preclinical advances, the clinical role of prophylactic approaches for BCBM is poorly investigated, and clinical features alone may not identify high-risk patients for BM to justify the toxicity associated with prophylactic therapies. The identification of tissue-based risk signatures could help overcome this. Prophylactic cranial irradiation (PCI) slowed disease progression resulting in survival benefit in small-cell lung cancer (SCLC), providing the rationale for application in BC. Currently, a randomized phase III trial is investigating the potential of a prophylactic taxane/trastuzumab treatment alone or in combination with PCI (Table 2). Published case series and retrospective analyses, however, indicate that PCI and its benefit-to-risk ratio in BC patients at high risk for BM should be approached critically (Huang et al., 2009). A phase III trial comparing the EGFR/HER2 kinase inhibitor lapatinib plus capecitabine versus capecitabine alone in patients with locally advanced or metastatic HER2-positive breast cancer that were previously treated with taxane-, anthracycline-or trastuzumab-containing therapies revealed a significant reduction in the number of cases with CNS involvement as the first site of progression. These results indicate that the addition of the receptor tyrosine kinase (RTK) inhibitor to chemotherapy can improve the prevention of brain metastases (Cameron et al., 2008). In agreement with the efficacy of this combination on preventing brain lesions, significant responses were witnessed in patients with established disease (Bachelot et al., 2013).
Table 2.
Ongoing clinical trials in patients with BCBM.
| Treatment | Population | Phase | Primary endpoint | Status | Results/Comments | Clinicaltrials.gov ID | |
|---|---|---|---|---|---|---|---|
| PREVENTION | PCI | HER2-positive | I | Safety, neurocognitive function | Completed | NCT00916877 | |
| PCI | HER2-positive | III | Incidence of BM in women receiving trastuzumab and chemotherapy | Completed | NCT00639366 | ||
| Lapatinib + capecitabine | HER2-positive | III | CNS metastasis as site of first relapse | Active | Not recruiting | NCT00820222 | |
| TREATMENT | Trastuzumab + BKM120 | HER2-positive | I | Adverse events and DLT | Active | Not yet recruiting | NCT01132664 |
| Trastuzumab + everolimus + vinorelbine | HER2-positive | II | Intracranial ORR | Recruiting | NCT01305941 | ||
| Trastuzumab + ARRY-380 | HER2-positive | I | Maximum tolerated dose of ARRY-380 with trastuzumab | Recruiting | NCT01921335 | ||
| Trastuzumab + GRN1005 | HER2-positive | II | Intracranial ORR | Recruiting | NCT01480583 | ||
| Lapatinib | HER2-positive | II | CNS ORR | Completed | NCT00098605 | ||
| Lapatinib + WBRT | Breast and lung cancer brain metastases | II | Response rate | Recruiting | NCT01218529 | ||
| Lapatinib + WBRT | HER2-positive | II | Response | Recruiting | NCT01622868 | ||
| Lapatinib + temozolomide | HER2-positive | I | MTD and DLT | Completed | NCT00614978 | ||
| Lapatinib + WBRT | HER2-positive | I | MTD | Completed | MTD of lapatinib when combined with WBRT: 1250 mg, PFS 4.8 months, OS 19 months | NCT00470847 | |
| Lapatinib + capecitabine | HER2-positive | II | ORR | Completed | NCT00967031 | ||
| Afatinib +/− vinorelbine | HER2-positive | II | Benefit at 12 weeks | Completed | NCT01441596 | ||
| Neratinib | HER2-positive | II | ORR | Recruiting | NCT01494662 | ||
| Bevacizumab + carboplatin | All subtypes | II | CNS ORR | Active | Not recruiting | NCT01004172 | |
| Bevacizumab + cisplatin + etoposide | All subtypes | II | ORR | Completed | NCT01281696 | ||
| ANG1005 | HER2-positive | II | Intracranial ORR | Recruiting | NCT02048059 | ||
| INIPARIB + irinotecan | Triple-negative | II | Efficacy as measured by intra or extracranial TTP | Completed | PARP inhibitor in combination with chemotherapy | NCT01173497 | |
| BKM120 + capecitabine | Triple-negative | II | Clinical benefit rate | Recruiting | NCT02000882 | ||
| Stereotactic radiotherapy + HER-2 therapy | HER2-positive | II | 6-month distant brain relapse rate | Not yet open for recruitment | NCT01924351 | ||
| Hippocampus-sparing WBRT | All subtypes | III | Cognitive toxicity | Recruiting | NCT01942980 | ||
| Bevacizumab + etoposide + cisplatin followed by WBRT | All subtypes | II | Brain specific PFS | Not yet open for recruitment | NCT02185352 | ||
| WBRT + temozolomide | Breast and lung cancer brain metastases | II | ORR | Not yet open for recruitment | NCT02133677 | ||
| Lapatinib following cranial radiotherapy | HER2-positive | II | Response to lapatinib | Ongoing | Not recruiting | NCT00263588 | |
| WBRT + sorafenib | All subtypes | I | MTD | Recruiting | NCT01724606 | ||
| Cabozantinib +/− trastuzumab | All subtypes | II | ORR | Not yet open for recruitment | NCT02260531 | ||
| Ado-trastuzumab emtansine+ Radiotherapy | HER2-positive | I | Optimal sequence | Ongoing | Not recruiting | NCT02135159 | |
| Abemaciclib | Hormone receptor positive | II | ORR | Not yet open for recruitment | NCT02308020 | ||
| Capecitabine + Radiotherapy | All subtypes | II | Best objective CNS response | Completed | NCT00977379 | ||
| Cabazitaxel | Breast cancer and lung cancer | II | Objective tumor response | Not yet open for recruitment | NCT02166658 | ||
| KD019 + trastuzumab | HER2 positive | Ib/IIa | Safety/Tolerability | Recruiting | NCT02154529 | ||
| Epothilone B | All subtypes | II | CNS PFS | Completed | NCT00450866 | ||
| TPI 287 | All subtypes | II | ORR | Recruiting | NCT01332630 |
PCI, prophylactic cranial irradiation; WBRT, whole brain radiotherapy; BM, brain metastases; CNS, central nervous system; MTD, maximum tolerated dose; DLT, dose-limiting toxicity; TTP, time to progression; PFS, progression-free survival; ORR, overall response rate.
BCBM can be diagnosed many years after the initial diagnosis of the disease. This should be taken into consideration when investigating the role of prophylactic therapies. Recent studies revealed that single cells or clusters of disseminated breast cancer cells might remain quiescent for a long time. This latency can be induced by endothelial-derived thrombospondin-1 suggesting the perivascular niche can regulate breast tumor dormancy (Ghajar et al., 2013). The dormancy of disseminated breast cancer cells depends on the histologic subtype (Aversa et al., 2014) and poses a major challenge for prophylactic strategies, which may cause severe adverse effects. For example, cranial irradiation is associated with acute, subacute and late/chronic toxicity. In the acute phase radiation therapy can cause vasogenic edema, resulting in headache, nausea or neurologic deficits. Subacute encephalopathy may appear up to 6 months post treatment and can progress into chronic cerebral dysfunction. The latter can be irreversible and cause neurocognitive deficits, leuko-encephalopathy, cerebral atrophy or even radiation induced necrosis (Dietrich et al., 2008; Le Pechoux et al., 2011). Markers for neuronal injury can be detected in the cerebrospinal fluid of patients after PCI, despite the moderate radiation doses used (Kalm et al., 2014). Similarly, RTKs such as lapatinib can induce significant toxicities, including cutaneous, gastrointestinal and hematologic side effects, as well as fatigue (Crown et al., 2013). The long latency for BM makes the determination of the optimal time point for initiation of PCI or prophylaxis using drugs difficult. This must be taken into account when designing prophylactic clinical trials.
Is the brain microenvironment the Achilles’ heel of modern therapies?
The anti-HER2 antibody trastuzumab, one of the most widely prescribed targeted therapeutics, is an essential component in the treatment of HER2-positive breast cancer. Although trastuzumab is effective for systemic disease, its efficacy against BM remains controversial. Differential sensitivity to trastuzumab between BM and mammary fat pad tumors is unambiguous (Kodack et al., 2012). Meta-analysis of the phase III adjuvant trials NSABP B31, NCCTG N9831, HERA and PACS 04 revealed a higher incidence for cerebral metastasis after adjuvant treatment with trastuzumab (Olson et al., 2013). This is associated with controlled systemic, extracranial disease (Bendell et al., 2003), supporting the hypothesis that the enhanced risk for BM after adjuvant trastuzumab treatment is due to improved systemic control. Clinical evidence for the efficacy of trastuzumab against established BM is limited, mainly due to the lack of prospective data in this setting. Retrospective analyses indicate a trend towards improved outcome (Bartsch et al., 2007), however it remains unclear whether the benefit is due to improved systemic control or drug efficacy against the cerebral lesions. In either case, the relative risk of the brain as the first site of relapse is significantly increased in HER2-positive patients treated with trastuzumab (Olson et al., 2013).
The limited efficacy of trastuzumab against BM is often attributed to an inadequate penetration through the BBB. Based on its presumed ability to better penetrate the BBB than trastuzumab, lapatinib, a small molecule kinase inhibitor of EGFR and HER2, was evaluated in BCBM. Lapatinib exhibited efficacy in a preclinical prevention model of HER2-overexpressing BCBM (Gril et al., 2008), leading to prospective clinical trials. In breast cancer patients with HER2-positive BM that progressed after WBRT, lapatinib showed very modest activity as a single agent (Lin et al., 2008; Lin et al., 2009). In one study of 39 patients, there was only 1 partial response (2.6%) at 16 weeks after lapatinib initiation; meanwhile, 4 of 16 patients (25%) with non-CNS disease achieved a partial response, but were eventually taken off due to CNS progression (Lin et al., 2008). In a separate study, lapatinib monotherapy showed a response rate of 6% (15 of 237) in a similar subset of patients (Lin et al., 2009). The success of lapatinib and capecitabine for systemic disease along with its activity in preventing brain metastasis led to its inclusion in patients with established BM. Interestingly, the addition of capecitabine to lapatinib increased response rates to 20% (Lin et al., 2009). Consistent with this data, the combination of lapatinib and capecitabine, before WBRT, in newly diagnosed BM (LANDSCAPE trial) revealed a CNS objective response rate of 67% (Bachelot et al., 2013). Further analysis indicated that the response correlated with a decrease in circulating tumor cells during treatment (Pierga et al., 2013). The mechanism for the significant efficacy of the combination treatment regimen remains unclear, but clearly capecitabine is active in this setting. As with trastuzumab, the question of whether brain metastatic resistance to lapatinib monotherapy is due in part to a lack of drug penetration remains unresolved, as this parameter has not been thoroughly investigated in the clinical setting.
Blood-brain barrier: time to rethink its importance in treating BM?
The BBB and expression of BBB transporters are thought to diminish the concentration of systemic therapy available to brain metastatic lesions (Deeken and Loscher, 2007). However, the blood-tumor barrier (BTB) is leakier than the BBB and permits delivery in brain lesions especially at later stages of disease (Murrell et al., 2014). The extent of BBB disruption varies amongst tumor subtypes (Yonemori et al., 2010). Consistent with a disrupted BBB, significant responses to chemotherapy are reported. Rosner et al. (Rosner et al., 1986) found a brain specific objective response rate of 50% in 100 breast cancer patients with symptomatic BM treated with a variety of chemotherapies. These findings were supported in subsequent studies (Stemmler and Heinemann, 2008), and include activity of capecitabine monotherapy, which was shown to achieve clinically relevant concentrations in non-irradiated human BCBM (Morikawa et al., 2013). Despite these reports suggesting a direct activity of chemotherapy in BM similar to what is observed in extracranial disease, chemotherapy is generally prescribed secondary to surgery or radiotherapy.
Diminished cerebrospinal fluid (CSF) concentration, compared to plasma levels, was cited as a mechanism of trastuzumab ineffectiveness due to an inadequate penetration through the BBB (Stemmler et al., 2007). However, the CSF represents a separate compartment from the brain parenchyma, separated from the parenteral circulation by the blood-CSF barrier and from the brain parenchyma by the glia limitans. In support of inadequate trastuzumab penetration as a reason for its ineffectiveness, increased delivery of trastuzumab via phosphodiesterase inhibition-induced BBB disruption enhanced its efficacy in a murine intracranial model (Hu et al., 2010). Consistent with the BTB being leaky, PET-based clinical studies demonstrated an accumulation of trastuzumab in human BCBM despite its high molecular weight (Tamura et al., 2013). It remains unclear, however, whether the concentration of trastuzumab achieved in the BM setting is sufficient to slow tumor growth (Lampson L.A, 2011). Recent clinical findings describing the efficacy of antibody-based therapy in BCBM suggest sufficient concentrations are achieved in brain metastatic lesions – these include bevacizumab (Lin et al., 2013; Lu et al., 2012) and trastuzumab-DM1 [(Bartsch et al., 2014; Krop et al., 2014; Yamamoto et al., 2012), see “Targeting the brain microenvironment for successful therapy” and “Future approaches”]. As with trastuzumab, the question of achieving an adequate concentration of lapatinib within the BM lesion remains unanswered. In preclinical models, lapatinib accumulated in brain lesions at higher concentrations than the normal brain, but at significantly lower levels than extracranial disease (Kodack et al., 2012; Taskar et al., 2012). Still, the concentrations achieved were significantly higher than its IC50 in vitro, and in our study led to significant inhibition of HER2 phosphorylation and downstream signaling. Human data indicate clinically relevant lapatinib concentrations are achieved in a number of BCBM, though a significant variation amongst them exists (Morikawa et al., 2013).
Strategies to enhance the delivery of therapeutics into the CNS have been actively pursued, and are beginning to undergo clinical evaluation. Radiation is known to disrupt the BBB (Brown et al., 2005). Hence, the efficacy of trastuzumab, lapatinib, and/or bevacizumab in combination with radiotherapy is currently being investigated in clinical trials (see Table 2). Other approaches to physically breakdown the BBB include infusion of a hyperosmotic agent, focused ultrasound, or treatment with bradykinin analogs (Eichler et al., 2011). One concern about disruption of the BBB is the potential of allowing circulating cancer cells more ready access to the brain parenchyma, thus potentially initiating new brain lesions. Furthermore, chronic BBB breakdown is associated with the accumulation of serum proteins and peripherally derived neurotoxic macromolecules, ultimately leading to neuronal degenerative changes (Bell et al., 2010). In addition to BBB disruption, other methods have been studied to enhance drug movement across the BBB. Taking advantage of the endogenously expressed BBB receptor low-density lipoprotein receptor-related protein (LRP-1), peptide-chemotherapy conjugates were designed to achieve superior delivery into preclinical brain metastasis models (Thomas et al., 2009). This class of agents is currently being tested in phase II clinical trials (NCT01480583 and NCT02048059, Table 2.) Other receptors that have been exploited for receptor-mediated BBB transcytosis include the transferrin, insulin and insulin-like growth factor-1 receptors (Eichler et al., 2011).
Another issue that remains clinically unexplored is whether active drug efflux mechanisms compromise the delivery of therapeutics in BM. While preclinical data indicate that p-glycoprotein (p-gp) mediated efflux kinetics are similar between normal BBB and BTB (Adkins et al., 2013), clinical data suggest p-gp expression in metastatic brain tumors is similar to that of primary, extracranial tumors and decreased compared to primary brain tumors (Gerstner and Fine, 2007). In a murine model, a non-p-gp substrate HER2/EGFR kinase inhibitor displayed modest but significantly better control of brain tumors compared with lapatinib (Nakayama et al., 2013), supporting the role of active drug efflux on pharmacokinetics and efficacy. While inhibitors of p-gp and other BBB transporters have increased drug concentrations in the murine CNS, knowledge of their efficacy on tumor growth of BCBM is lacking.
Targeting the brain microenvironment for successful therapy
In preclinical models, BM from breast cancer exhibits higher microvascular density than their respective primary tumors (Monsky et al., 2002). These data underscore the crucial role of the microenvironment in shaping and defining biological properties of the tumor and suggest that BM may be more reliant on blood vessels than primary tumors. Indeed, angiogenesis is required for efficient colonization and growth of breast cancer cells in the brain, as inhibiting vascular endothelial growth factor (VEGF) receptor activation reduces brain metastatic growth of brain-tropic breast cancer cell variants through a reduction in angiogenesis (Kim et al., 2004). Despite the lack of an overall survival benefit with the anti-VEGF antibody bevacizumab (in combination with chemotherapy) in breast cancer patients with extracranial disease (Brufsky et al., 2011a; Robert et al., 2011), case series suggest that patients with BM may benefit from the addition of bevacizumab to systemic chemotherapy (Yamamoto et al., 2012). Furthermore, preliminary data from phase II clinical trials show objective response rates of up to 75% with the combination of bevacizumab and chemotherapy (Lin, 2013; Lu YS, 2012). This is the subject of investigation in an ongoing clinical trial in BCBM patients (http://www.clinicaltrials.gov identifier NCT01004172, Table 2). These findings raise the questions of whether BM are more reliant on angiogenesis than extracranial tumors and/or if the brain endothelium is more reliant on VEGF than the systemic vasculature. While we hypothesize the increased vascularity of BM is due to enhanced angiogenesis, it could also result from vessel co-option, a known mechanism of resistance to anti-VEGF therapies in primary brain tumors (di Tomaso et al., 2011; Jain, R.K. 2014). We will be better positioned to answer these questions as findings from the clinical trials become available.
The crosstalk between the HER2 and VEGF pathways provides a compelling rationale for combined approaches with HER2 targeted agents and anti-VEGF drugs. Despite the lack of a progression-free survival benefit with bevacizumab plus trastuzumab and docetaxel in patients with HER2-positive locally recurrent or metastatic extracranial disease (Gianni et al., 2013), there are reasons to be optimistic for this combination in the setting of BM. The combination of trastuzumab or lapatinib with antibodies targeting VEGF receptor-2 was very effective in preclinical BCBM models (Kodack et al., 2012), and preliminary analysis of a phase II clinical trial of bevacizumab, trastuzumab, and carboplatin show objective response rates of up to 75% (Lin, 2013; Lu YS, 2012). This study is currently ongoing (NCT01004172, see Table 2), and will determine if a phase III trial is warranted. Furthermore, the combination of anti-VEGF therapy and dual HER2 inhibition (trastuzumab plus lapatinib) showed the best activity in preclinical models, and is well tolerated and active in heavily pretreated patients, including those with brain lesions (Falchook et al., 2013; Kodack et al., 2012).
Our studies have also shown that trastuzumab can induce vessel normalization in preclinical models of HER2 overexpressing BCBM (Izumi et al., 2002), a feature that is associated with improved tumor oxygenation and radiosensitization (Winkler et al., 2004). This finding provides a rationale to combine trastuzumab with radiotherapy, and we await the results of a completed phase II trial designed to test this combination (NCT01363986, see Table 2).
Future approaches for treating BCBM
Additional approaches focus on the use of novel targeting agents for BCBM. Recently the novel antibody-chemotherapy conjugate ado-trastuzumab emtansine (T-DM1) was approved for the treatment of HER2-positive breast cancer. Approval was based on the prospective phase III trial EMILIA, which revealed that T-DM1 was superior to lapatinib and capecitabine in patients with disease progression after trastuzumab (Verma et al., 2012). If reduced efficacy of trastuzumab in BM is not due solely to inefficient delivery, but instead due to acquired or microenvironment-mediated activation of alternative signaling pathways, T-DM1 would be expected to be effective in these patients. This hypothesis is supported by preclinical findings (Askoxylakis et al., unpublished data), case reports (Bartsch et al., 2014) and a subgroup analysis (asymptomatic brain metastases) in a randomized, open-label, phase III trial of previously treated (physician’s choice) metastatic HER2-positive patients (Krop et al., 2014). Furthermore, new generation ErbB family inhibitors, more potent and specific than lapatinib (neratinib and afatinib), showed significant responses in single cases of BCBM (Yap et al., 2010). Prospective clinical trials (NCT01494662 and NCT01441596) investigating the efficacy of these drugs in patients with BCBM are currently accruing (see Table 2). In addition, the elucidation of mechanisms of de novo or acquired resistance to anti-HER2 therapy in systemic disease led to the evaluation of downstream HER2 signaling inhibitors in BM, including the PI3K inhibitor BKM120 and the mTOR inhibitor everolimus, either alone or in combination with trastuzumab (see Table 2). Furthermore, the HER2 family member HER3, critical for HER2 downstream signaling, is enriched in human BM compared to matched primary breast tumors (Da Silva et al., 2010). Indeed, inhibiting HER3 activity enhances the efficacy of HER2-targeted therapies in preclinical models of BCBM (Kodack et al., unpublished data). The role of immunotherapy in cancer treatment is a subject of major interest in recent years, but not much is known with regard to BCBM. Knowledge from other malignancies, such as the activity of ipilimumab in patients with cerebral metastases from malignant melanoma (Margolin et al., 2012), suggests that immune system modulation might be of promise, and emphasizes the necessity for studies in this direction. Brain metastatic lesions from breast cancer contain activated microglia, and, although the brain is considered immune privileged, preclinical studies clearly showed that the peripheral immune system enters the brain parenchyma after CNS insult (Ousman and Kubes, 2012). Indirect activation of NK cells, CD4+ and CD8+ T cells, through CpG oligodeoxynucleotide treatment, prevented brain metastasis of murine breast carcinoma cells injected into syngeneic mice, but failed to slow the growth of established BM (Xiong et al., 2008).
Conclusions/Perspective
Better therapies for BCBM are needed. The efficacy of existing therapies, used for the treatment of systemic disease, is largely unclear in patients with BM, mainly due to the fact that the presence of BM served as an exclusion criterion for most prospective trials, including the phase III studies TH3RESA, CLEOPATRA, and EMILIA. Therefore, to better understand the efficacy of standard targeted therapies, patients with BM must be included in clinical trials. Furthermore, analysis of clinical tissue to confirm preclinical data and determine clinical evidence of suspected resistance mechanisms is necessary. While a number of preclinical reports identify genes that mediate BM, most describe those necessary for the initial steps of brain metastatic colonization. This provides essential information and rationale for clinical applications on metastasis prevention; however, the central issue of effective treatment of established BM remains open. In this respect there are unanswered questions that need to be addressed: Is treatment resistance of BM due to a lack of drug penetration into the brain lesion? Has the brain metastatic cancer cell evolved to evade the same therapy to which its predecessors are sensitive? Does the brain microenvironment provide factors that enable cancer cells to become resistant, and if so, what are these crucial determinants of resistance? What is the role of intratumoral heterogeneity? How can microenvironment-targeted therapies, such as anti-angiogenic or immunotherapy, improve the therapeutic efficacy?
Despite the lack of definitive answers to these questions, recent data provide some insight that could drive future approaches. The reduced efficacy of antibody-based therapies in the brain has been attributed to the decreased permeability through the BBB, however the efficacy of bevacizumab or T-DM1 suggests adequate penetration of antibodies into the brain metastatic lesion and encourages the investigation of other large molecule therapies in the BM setting. If therapies are indeed achieving adequate concentrations within BM then resistance could be attributed to non-pharmacokinetic mechanisms. Specific features of the brain microenvironment and current biological aspects of the seed and soil hypothesis have been implicated in treatment resistance. This seems, however, not to be a universal phenomenon, but instead may be dependent on the nature of the parental lesion. Whereas the majority of HER2-positive BCBM are resistant to targeted therapies, the response rates of lung cancer or melanoma BM to EGFR or BRAF inhibitors, respectively, are similar to extracranial disease (Lombardi et al., 2014). This suggests a unique crosstalk between the brain microenvironment and biological features of breast cancer cells that need to be investigated in further detail. Successful therapies may consist of combinatorial approaches targeting the tumor stroma in addition to the cancer cell, while limiting neuronal damage. Finally, intratumoral heterogeneity should be taken into account. Discordance between primary disease and metastatic lesions has been described (Niikura et al., 2012). This heterogeneity makes the interactions between tumor cells and the microenvironment more complicated, and emphasizes the need to select an appropriate therapeutic strategy based on characteristics of the metastases rather than the primary tumor. In conclusion, despite major preclinical and clinical progress in the characterization, prevention and management of BM, the multitude and complexity of the remaining questions to be answered will require a tighter integration between bench and bedside.
Acknowledgments
We thank M. Badeaux and S. Goel for their helpful suggestions and editing. This work was supported by US Department of Defense Breast Cancer Research Innovator Award W81XWH-10-1-0016 (to R.K.J.); US National Cancer Institute Grants R01-CA126642 (to R.K.J.), P01-CA080124 (to R.K.J. and D.F.), R01-CA096915 (to D.F.), and Breast SPORE grant P50 CA098131 (to C.L.A.); Federal Share Proton Beam Program Income (to R.K.J.); and German Research Foundation Grant (Deutsche Forschungsgemeinschaft, DFG) AS 422-2/1 (to V.A.). R.K.J. received consultant fees from Enlight, Ophthotech, and SynDevRx. R.K.J. owns equity in Enlight, Ophthotech, SynDevRx, and XTuit and serves on the Board of Directors of XTuit and the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, and the Tekla Healthcare Opportunities Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins CE, Mittapalli RK, Manda VK, Nounou MI, Mohammad AS, Terrell TB, Bohn KA, Yasemin C, Grothe TR, Lockman JA, Lockman PR. P-glycoprotein mediated efflux limits substrate and drug uptake in a preclinical brain metastases of breast cancer model. Frontiers in Pharmacology. 2013;4:136. doi: 10.3389/fphar.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Aversa C, Rossi V, Geuna E, Martinello R, Milani A, Redana S, Valabrega G, Aglietta M, Montemurro F. Metastatic breast cancer subtypes and central nervous system metastases. Breast. 2014;23:623–628. doi: 10.1016/j.breast.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, Jimenez M, Le Rhun E, Pierga JY, Goncalves A, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncology. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- Bartsch R, Berghoff AS, Preusser M. Breast cancer brain metastases responding to primary systemic therapy with T-DM1. Journal of Neuro-Oncology. 2014;116:205–206. doi: 10.1007/s11060-013-1257-5. [DOI] [PubMed] [Google Scholar]
- Bartsch R, Rottenfusser A, Wenzel C, Dieckmann K, Pluschnig U, Altorjai G, Rudas M, Mader RM, Poetter R, Zielinski CC, Steger GG. Trastuzumab prolongs overall survival in patients with brain metastases from Her2 positive breast cancer. Journal of Neuro-Oncology. 2007;85:311–317. doi: 10.1007/s11060-007-9420-5. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, Bunnell C, Rue M, Gelman R, Winer E. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiation Research. 2005;164:662–668. doi: 10.1667/rr3453.1. [DOI] [PubMed] [Google Scholar]
- Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, Rugo HS. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. Journal of Clinical Oncology. 2011a;29:4286–4293. doi: 10.1200/JCO.2010.34.1255. [DOI] [PubMed] [Google Scholar]
- Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, Tudor IC, Wang LI, Brammer MG, Shing M, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clinical Cancer Research. 2011b;17:4834–4843. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, Chan S, Jagiello-Gruszfeld A, Kaufman B, Crown J, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Research and Treatment. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- Chen J, Lee HJ, Wu X, Huo L, Kim SJ, Xu L, Wang Y, He J, Bollu LR, Gao G, et al. Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Cancer research. 2014 doi: 10.1158/0008-5472.CAN-14-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown J, Kennedy MJ, Tresca P, Marty M, Espie M, Burris HA, DeSilvio M, Lau MR, Kothari D, Koch KM, Dieras V. Optimally tolerated dose of lapatinib in combination with docetaxel plus trastuzumab in first-line treatment of HER2-positive metastatic breast cancer. Annals of Oncology. 2013;24:2005–2011. doi: 10.1093/annonc/mdt222. [DOI] [PubMed] [Google Scholar]
- Da Silva L, Simpson PT, Smart CE, Cocciardi S, Waddell N, Lane A, Morrison BJ, Vargas AC, Healey S, Beesley J, et al. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Research: BCR. 2010;12:R46. doi: 10.1186/bcr2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clinical Cancer Research. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- di Tomaso E, Snuderl M, Kamoun WS, Duda DG, Auluck PK, Fazlollahi L, Andronesi OC, Frosch MP, Wen PY, Plotkin SR, et al. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Research. 2011;71:19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. The Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, Jain RK. Malignant cells facilitate lung metastasis by bringing their own soil. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases: translation to new therapies. Nature Reviews Clinical Oncology. 2011;8:344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchook GS, Moulder SL, Wheler JJ, Jiang Y, Bastida CC, Kurzrock R. Dual HER2 inhibition in combination with anti-VEGF treatment is active in heavily pretreated HER2-positive breast cancer. Annals of Oncology. 2013;24:3004–3011. doi: 10.1093/annonc/mdt395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DP, Palmieri D, Hua E, Hargrave E, Herring JM, Qian Y, Vega-Valle E, Weil RJ, Stark AM, Vortmeyer AO, Steeg PS. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clinical & Experimental Metastasis. 2008;25:799–810. doi: 10.1007/s10585-008-9193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DP, Subramanian P, Deshpande M, Graves C, Gordon I, Qian Y, Snitkovsky Y, Liewehr DJ, Steinberg SM, Paltan-Ortiz JD, et al. Opposing effects of pigment epithelium-derived factor on breast cancer cell versus neuronal survival: implication for brain metastasis and metastasis-induced brain damage. Cancer Research. 2012;72:144–153. doi: 10.1158/0008-5472.CAN-11-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. Journal of Clinical Oncology. 2007;25:2306–2312. doi: 10.1200/JCO.2006.10.0677. [DOI] [PubMed] [Google Scholar]
- Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, et al. The perivascular niche regulates breast tumour dormancy. Nature Cell Biology. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O, et al. AVEREL: a randomized phase III trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. Journal of Clinical Oncology. 2013;31:1719–1725. doi: 10.1200/JCO.2012.44.7912. [DOI] [PubMed] [Google Scholar]
- Gori S, Rimondini S, De Angelis V, Colozza M, Bisagni G, Moretti G, Sidoni A, Basurto C, Aristei C, Anastasi P, Crino L. Central nervous system metastases in HER-2 positive metastatic breast cancer patients treated with trastuzumab: incidence, survival, and risk factors. The Oncologist. 2007;12:766–773. doi: 10.1634/theoncologist.12-7-766. [DOI] [PubMed] [Google Scholar]
- Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, Liewehr DJ, Steinberg SM, Merino MJ, Rubin SD, Steeg PS. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. Journal of the National Cancer Institute. 2008;100:1092–1103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Ljubimova JY, Inoue S, Konda B, Patil R, Ding H, Espinoza A, Wawrowsky KA, Patil C, Ljubimov AV, Black KL. Phosphodiesterase type 5 inhibitors increase Herceptin transport and treatment efficacy in mouse metastatic brain tumor models. PloS One. 2010;5:e10108. doi: 10.1371/journal.pone.0010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Alrefae M, Langleben A, Roberge D. Prophylactic cranial irradiation in advanced breast cancer: a case for caution. International Journal of Radiation Oncology, Biology, Physics. 2009;73:752–758. doi: 10.1016/j.ijrobp.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- Jain RK. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell. 2014 doi: 10.1016/j.ccell.2014.10.006. http://dx.doi.org/10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed]
- Kalm M, Abel E, Wasling P, Nyman J, Hietala MA, Bremell D, Hagberg L, Elam M, Blennow K, Bjork-Eriksson T, Zetterberg H. Neurochemical evidence of potential neurotoxicity after prophylactic cranial irradiation. International Journal of Radiation Oncology, Biology, Physics. 2014;89:607–614. doi: 10.1016/j.ijrobp.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. Journal of Clinical Oncology. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nature Medicine. 2010;16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- Kim LS, Huang S, Lu W, Lev DC, Price JE. Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clinical & Experimental Metastasis. 2004;21:107–118. doi: 10.1023/b:clin.0000024761.00373.55. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, Maya M, He J, Kim SW, Weihua Z, et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia. 2011;13:286–298. doi: 10.1593/neo.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Choi HJ, Lee HJ, He J, Wu Q, Langley RR, Fidler IJ, Kim SJ. Role of the endothelin axis in astrocyte-and endothelial cell-mediated chemoprotection of cancer cells. Neuro-Oncology. 2014 Jul 9; doi: 10.1093/neuonc/nou128. pii: nou128. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. Journal of Clinical Oncology. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AM, Song Y, Farrar CT, Huang Y, Ager E, Kamoun W, et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3119–3127. doi: 10.1073/pnas.1216078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krop IE, Kim SB, Gonzalez-Martin A, LoRusso PM, Ferrero JM, Smitt M, Yu R, Leung AC, Wildiers H T. R. s collaborators. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncology. 2014;15:689–699. doi: 10.1016/S1470-2045(14)70178-0. [DOI] [PubMed] [Google Scholar]
- Lampson LA. Monoclonal antibodies in neuro-oncology: Getting past the blood-brain barrier. MAbs. 2011;3:153–160. doi: 10.4161/mabs.3.2.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pechoux C, Laplanche A, Faivre-Finn C, Ciuleanu T, Wanders R, Lerouge D, Keus R, Hatton M, Videtic GM, Senan S, et al. Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99-01, EORTC 22003-08004, RTOG 0212 and IFCT 99-01) Annals of Oncology. 2011;22:1154–1163. doi: 10.1093/annonc/mdq576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, Harris GJ, Bullitt E, Van den Abbeele AD, Henson JW, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. Journal of Clinical Oncology. 2008;26:1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin NU, Dieras V, Paul D, Lossignol D, Christodoulou C, Stemmler HJ, Roche H, Liu MC, Greil R, Ciruelos E, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clinical Cancer Research. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- Lin NU, Gelman RS, Younger WJ, Sohl J, Freedman RA, Sorensen AG, Bullitt E, Harris GH, Morganstern D, Schneider BP, Krop IE, Winer EP. Phase II trial of carboplatin (C) and bevacizumab (BEV) in patients (pts) with breast cancer brain metastases (BCBM) Journal of Clinical Oncology. 2013:31. [Google Scholar]
- Lombardi G, Di Stefano AL, Farina P, Zagonel V, Tabouret E. Systemic treatments for brain metastases from breast cancer, non-small cell lung cancer, melanoma and renal cell carcinoma: An overview of the literature. Cancer Treatment Reviews. 2014;40:951–959. doi: 10.1016/j.ctrv.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. American Journal of Pathology. 2010;176:2958–2971. doi: 10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie E, Chen XF, Coomes A, Ji K, Tsirka S, Chen EI. Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene. 2013;32:4064–4077. doi: 10.1038/onc.2012.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YS, CW, Lin CH, Tseng LM, Yeh DC, Wu PF, Chen BB, Chao TC, Tsai YF, Huang SM, Shih TTF, Cheng AF. Bevacizumab, etoposide, and cisplatin (BEEP) in brain metastases of breast cancer progressing from radiotherapy: results of the first stage of a multicenter phase II study. Journal of Clinical Oncology. 2012:30. [Google Scholar]
- Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncology. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- Monsky WL, Mouta Carreira C, Tsuzuki Y, Gohongi T, Fukumura D, Jain RK. Role of host microenvironment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clinical Cancer Research. 2002;8:1008–1013. [PubMed] [Google Scholar]
- Morikawa A, Peereboom DM, Smith QR, Thorsheim H, Lockman PR, Simmons AJ, Weil RJ, Tabar V, Steeg PS, Seidman AD. Clinical evidence for drug penetration of capecitabine and lapatinib uptake in resected brain metastases from women with metastatic breast cancer. Journal of Clinical Oncology. 2013:31. [Google Scholar]
- Murrell DH, Foster PJ, Chambers AF. Brain metastases from breast cancer: lessons from experimental magnetic resonance imaging studies and clinical implications. Journal of Molecular Medicine. 2014;92:5–12. doi: 10.1007/s00109-013-1108-z. [DOI] [PubMed] [Google Scholar]
- Nakayama A, Takagi S, Yusa T, Yaguchi M, Hayashi A, Tamura T, Kawakita Y, Ishikawa T, Ohta Y. Antitumor Activity of TAK-285, an Investigational, Non-Pgp Substrate HER2/EGFR Kinase Inhibitor, in Cultured Tumor Cells, Mouse and Rat Xenograft Tumors, and in an HER2-Positive Brain Metastasis Model. Journal of Cancer. 2013;4:557–565. doi: 10.7150/jca.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neman J, Choy C, Kowolik CM, Anderson A, Duenas VJ, Waliany S, Chen BT, Chen MY, Jandial R. Co-evolution of breast-to-brain metastasis and neural progenitor cells. Clinical & Experimental Metastasis. 2013;30:753–768. doi: 10.1007/s10585-013-9576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura N, Liu J, Hayashi N, Mittendorf EA, Gong Y, Palla SL, Tokuda Y, Gonzalez-Angulo AM, Hortobagyi GN, Ueno NT. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. Journal of Clinical Oncology. 2012;30:593–599. doi: 10.1200/JCO.2010.33.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EM, Abdel-Rasoul M, Maly J, Wu CS, Lin NU, Shapiro CL. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Annals of Oncology. 2013:1526–1533. doi: 10.1093/annonc/mdt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nature Neuroscience. 2012;15:1096–1101. doi: 10.1038/nn.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ES, Kim SJ, Kim SW, Yoon SL, Leem SH, Kim SB, Kim SM, Park YY, Cheong JH, Woo HG, et al. Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17456–17461. doi: 10.1073/pnas.1114210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierga JY, Bidard FC, Cropet C, Tresca P, Dalenc F, Romieu G, Campone M, Mahier Ait-Oukhatar C, Le Rhun E, Goncalves A, et al. Circulating tumor cells and brain metastasis outcome in patients with HER2-positive breast cancer: the LANDSCAPE trial. Annals of Oncology. 2013;24:2999–3004. doi: 10.1093/annonc/mdt348. [DOI] [PubMed] [Google Scholar]
- Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. Journal of Clinical Oncology. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- Rosner D, Nemoto T, Lane WW. Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer. 1986;58:832–839. doi: 10.1002/1097-0142(19860815)58:4<832::aid-cncr2820580404>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Sperduto PW, Kased N, Roberge D, Chao ST, Shanley R, Luo X, Sneed PK, Suh J, Weil RJ, Jensen AW, et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. Journal of Neuro-Oncology. 2013;112:467–472. doi: 10.1007/s11060-013-1083-9. [DOI] [PubMed] [Google Scholar]
- Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. Journal of Clinical Oncology. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmler HJ, Heinemann V. Central nervous system metastases in HER-2-overexpressing metastatic breast cancer: a treatment challenge. The Oncologist. 2008;13:739–750. doi: 10.1634/theoncologist.2008-0052. [DOI] [PubMed] [Google Scholar]
- Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anti-Cancer Drugs. 2007;18:23–28. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- Tamura K, Kurihara H, Yonemori K, Tsuda H, Suzuki J, Kono Y, Honda N, Kodaira M, Yamamoto H, Yunokawa M, et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. Journal of Nuclear Medicine. 2013;54:1869–1875. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- Taskar KS, Rudraraju V, Mittapalli RK, Samala R, Thorsheim HR, Lockman J, Gril B, Hua E, Palmieri D, Polli JW, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharmaceutical Research. 2012;29:770–781. doi: 10.1007/s11095-011-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, Mittapalli RK, Palmieri D, Steeg PS, Lockman PR, Smith QR. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharmaceutical Research. 2009;26:2486–2494. doi: 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, Massague J. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156:1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. The New England Journal of Medicine. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Gharagozlou S, Vengco I, Chen W, Ohlfest JR. Effective CpG immunotherapy of breast carcinoma prevents but fails to eradicate established brain metastasis. Clinical Cancer Research. 2008;14:5484–5493. doi: 10.1158/1078-0432.CCR-07-4139. [DOI] [PubMed] [Google Scholar]
- Yamamoto D, Iwase S, Tsubota Y, Sueoka N, Yamamoto C, Kitamura K, Odagiri H, Nagumo Y. Bevacizumab in the treatment of five patients with breast cancer and brain metastases: Japan Breast Cancer Research Network-07 trial. OncoTargets and Therapy. 2012;5:185–189. doi: 10.2147/OTT.S36515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TA, Vidal L, Adam J, Stephens P, Spicer J, Shaw H, Ang J, Temple G, Bell S, Shahidi M, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. Journal of Clinical Oncology. 2010;28:3965–3972. doi: 10.1200/JCO.2009.26.7278. [DOI] [PubMed] [Google Scholar]
- Yonemori K, Tsuta K, Ono M, Shimizu C, Hirakawa A, Hasegawa T, Hatanaka Y, Narita Y, Shibui S, Fujiwara Y. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu- positive breast cancer. Cancer. 2010;116:302–308. doi: 10.1002/cncr.24735. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ridgway LD, Wetzel MD, Ngo J, Yin W, Kumar D, Goodman JC, Groves MD, Marchetti D. The identification and characterization of breast cancer CTCs competent for brain metastasis. Science Translational Medicine. 2013;5:180, ra148. doi: 10.1126/scitranslmed.3005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Olsson Y. Reactions of astrocytes and microglial cells around hematogenous metastases of the human brain. Expression of endothelin-like immunoreactivity in reactive astrocytes and activation of microglial cells. Journal of the Neurological Sciences. 1995;134:26–32. doi: 10.1016/0022-510x(95)00227-9. [DOI] [PubMed] [Google Scholar]