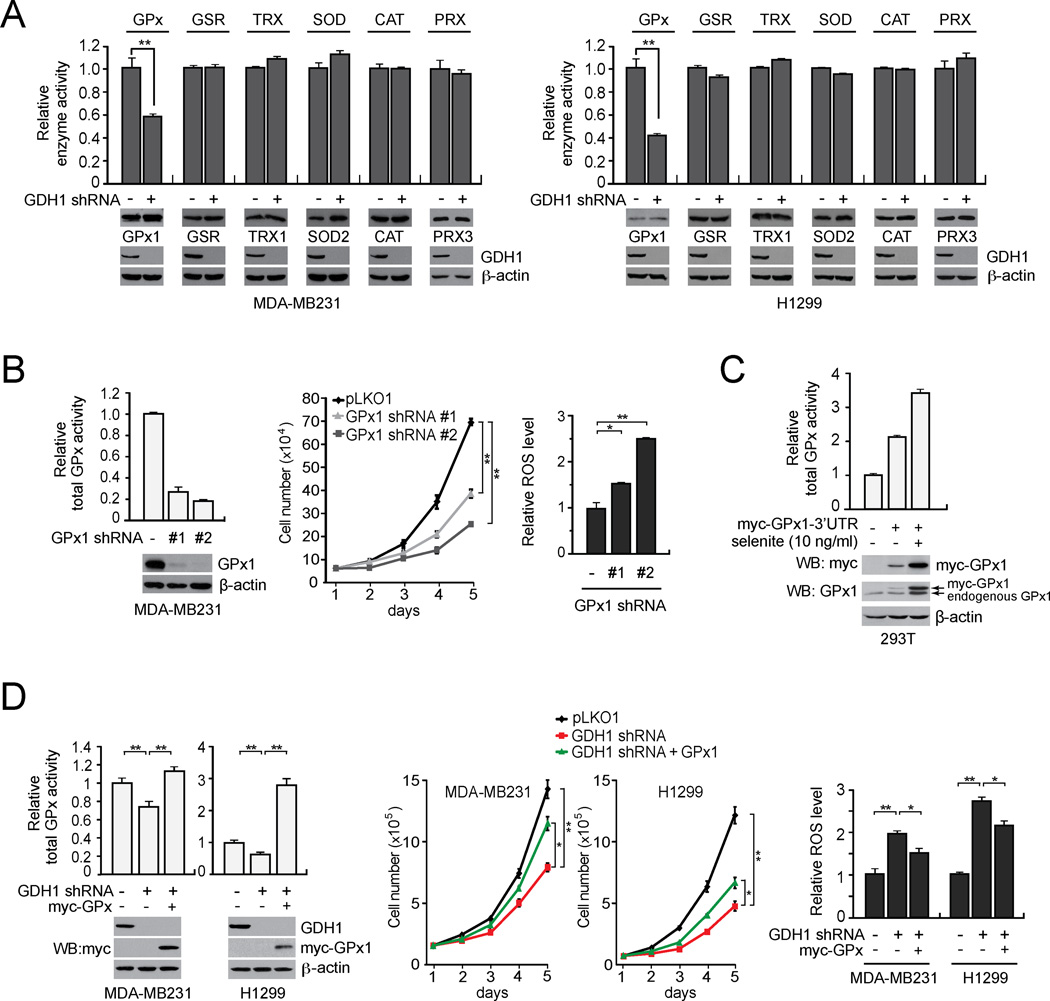
Figure 4. GDH1 contributes to redox homeostasis in part by regulating glutathione peroxidase (GPx) activity in cancer cells.
(A) Effect of GDH1 knockdown on the enzyme activity of GPx and other ROS scavenging enzymes including GSR, TRX, SOD, CAT and PRX in MDA-MB231 (left) and H1299 (right) cells. Western blots displaying the expression of GPx1, GSR, TRX1, SOD2, CAT, PRX3 and GDH1 in cells with GDH1 stable knockdown or an empty vector. β-actin was used as a loading control. (B) Effect of GPx1 knockdown on total GPx activity (left), cell proliferation (middle) and ROS (right) in MDA-MB231 cancer cells. Knockdown efficiency of GPx1 was determined by Western blotting. Cell proliferation rates and ROS levels were assessed by cell counting and carboxy-H2DCFDA detection, respectively. (C) Induction of GPx1 expression in 293T cells transduced with a GPx1 expression construct harboring a 3’UTR with a SECIS element that responds to selenite. Expression of myc tagged GPx1 was determined by immunoblotting using anti-myc and anti-GPx1 antibodies. (D) Effect of myc-GPx1 stable expression on the total cellular GPx activity (left), cell proliferation (middle) and ROS (right) in MDA-MB231 and H1299 cells with stable knockdown of GDH1. 10 ng/ml selenite was added in the culture media for all the assays. GDH1 knockdown and myc-GPx1 expression is shown by Western blot analyses. Data are mean ± SD from three replicates. p values were determined by a two-tailed Student’s t test (*0.01 < p < 0.05; **0.001 < p < 0.01). See also Figure S3.