Abstract
Leprosy is a chronic disease caused by Mycobacterium leprae, highly incapacitating, and with systemic involvement in some cases. Renal involvement has been reported in all forms of the disease, and it is more frequent in multibacillary forms. The clinical presentation is variable and is determined by the host immunologic system reaction to the bacilli. During the course of the disease there are the so called reactional states, in which the immune system reacts against the bacilli, exacerbating the clinical manifestations. Different renal lesions have been described in leprosy, including acute and chronic glomerulonephritis, interstitial nephritis, secondary amyloidosis and pyelonephritis. The exact mechanism that leads to glomerulonephritis in leprosy is not completely understood. Leprosy treatment includes rifampicin, dapsone and clofazimine. Prednisone and non-steroidal anti-inflammatory drugs may be used to control acute immunological episodes.
Keywords: Leprosy, Hansen disease, Kidney dysfunction, Chronic kidney disease, Glomerulonephritis
Abstract
A hanseníase é doença crônica causada pelo Mycobacterium leprae, altamente incapacitante e com envolvimento sistêmico em alguns casos. O envolvimento renal tem sido relatado em todas as formas da doença, sendo mais frequente nas formas multibacilares. A apresentação clínica é variável e determinada pela reação do sistema imunológico do hospedeiro ao bacilo. Durante o curso da doença podem ocorrer os chamados estados reacionais, nos quais o sistema imune reage contra o bacilo, exacerbando as manifestações clínicas. Diferentes lesões renais tem sido descritas na hanseníase, incluindo glomerulonefrites, nefrite intersticial, amiloidose secundária e pielonefrite. O mecanismo exato que leva à glomerulonefrite na hanseníase ainda não está completamente esclarecido. O tratamento da hanseníase inclui o uso de rifampicina, dapsona e clofazimina. Prednisona e antiinflamatórios não-hormonais podem ser usados no controle dos episódios imunológicos agudos.
INTRODUCTION
Leprosy is a chronic disease caused by Mycobacterium leprae, an acid-fast bacilli, intracellular parasite, with predilection to Schwann cell and skin. The disease is highly incapacitating, and systemic involvement is reported in some cases45. Renal involvement has been reported in all forms of the disease, and it is more frequent in multibacillary forms51. The present paper presents a review of the clinical and histopathological aspects of leprosy nephropathy.
EPIDEMIOLOGY: The number of leprosy patients is estimated to be between 10 and 15 million, distributed across more than 100 countries. In 2007, a total of 254,525 new cases were reported all over the world45. Brazil is considered as having a high endemicity index and is the country with the second highest number of cases, with 37,610 new cases registered in 200958. Leprosy prevalence in Brazil was reduced by 85%, going from 17 to 3.8 cases/10,000 population in the period between 1985 and 200135.
LEPROSY PATHOPHYSIOLOGY: Infected persons with M. leprae are thought not to develop clinical disease. M. leprae is slow growing and the incubation period is long at 2-12 years. The M. leprae has a high infective power, but low pathogenic power3. Person-to-person spread via nasal droplets is believed to be the main route of leprosy transmission. Most people with leprosy are non-infectious. Patients with lepromatous leprosy excrete M. leprae from their nasal mucosa and skin and are infectious before starting treatment with multidrug therapy. Contacts of these patients are, therefore, at increased risk of developing the disease. There may be a genetic predisposition to disease manifestation. Infection with M. leprae leads to chronic granulomatous inflammation in skin and peripheral nerves46. Single-nucleotide polymorphism (SNP) association studies showed a low lymphotoxin-α (LTA)-producing allele as a major genetic risk factor for early onset leprosy. Other SNPs to be associated with disease and/or the development of reactions in several genes, such as vitamin D receptor (VDR), TNF-α, IL-10, IFN-γ, HLA genes, and TLR1 have also been suggested6. The type of leprosy that patients develop is determined by their cell-mediated immune response to infection. Patients with tuberculoid disease have a good cell-mediated immune response and few lesions with no detectable mycobacteria. Patients with lepromatous leprosy are anergic towards M. leprae and have multiple lesions with mycobacteria present46. Schwann cells (SCs) are a major target for infection by M. leprae leading to nerve injury, demyelination, and consequent disability. Binding of M. leprae to SCs induces demyelination and loss of axonal conductance. Macrophages are one of the most abundant host cells to come in contact with mycobacteria. Phagocytosis of M. leprae by monocyte-derived macrophages can be mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and is regulated by protein kinase6. The inflammation present in nerves is driven by mycobacterial antigens that activate a destructive inflammatory immune response mediated by CD4+ cells and macrophages, and with involvement of multiple pro-inflammatory cytokines, such as tumor necrosis factor α46. In the tuberculoid lesions there is a predominance of CD4+ auxiliary T cells and Th1 cytokines such as IL-2 and IFN-gamma, while in lepromatous (Virchowian) lesions suppressant T cells, CD8+, and Th2 cytokines such as IL-4, IL-5 and IL-103 predominate. In the tuberculoid type, the exacerbation of cellular immunity and the production of pro-inflammatory cytokines (IL-1 and TNF-alpha) prevents the bacilli proliferation, but can cause injury to the host due to the lack of regulator factors. In the Virchowian type, the production of PGL-1 (phenolic glycolipid antigen-1) and LAM (lipoarabinomannan) antigens by the bacilli, inside macrophages, favors the escapade of the bacilli from the intramacrophage oxidation, because these antigens have a suppressant effect over macrophage activity, and then favors bacilli proliferation3.
CLINICAL MANIFESTATIONS: Leprosy is characterized by tegumentary lesions and nervous system injury. The clinical presentation is variable and is determined by the host immunologic system reaction to the bacilli. During the course of the disease there are the so called reactional states, in which the immune system reacts against the bacilli, exacerbating the clinical manifestations. There are two types of reactional states: reversal reaction (type I), which is more common in the paucibacillary forms, and erythema nodosum (type II), more common in multibacillary forms45. The disease is divided into four forms, according to the criteria established by the World Health Organization: indeterminate, tuberculoid, dimorphous and virchowian. The diagnosis and classification are based on clinical findings and complementary tests, such as baciloscopy, which allow the classification in multibacillary and paucibacillary.
EFFECTS OF IMMUNOSUPPRESSION, HIV AND TRANSPLANT IN LEPROSY: At the beginning of the HIV epidemic there was a fear that HIV infection could increase the risk of leprosy development or that the co-infection (HIV-leprosy) would cause a more severe disease46. This hypothesis was not confirmed, since some studies have shown that patients receiving highly active antiretroviral therapy are more likely to develop borderline tuberculoid leprosy than other types of leprosy46. HIV infection has not been reported to increase susceptibility to leprosy, impact on immune response to M. leprae, or to have a significant effect on the pathogenesis of neural or skin lesions to date. The initiation of antiretroviral treatment has been reported to be associated with activation of subclinical M. leprae infection and exacerbation of existing leprosy lesions (type I reaction) likely as part of immune reconstitution inflammatory syndrome6. Leprosy has also been reported to occur after organ transplantations, but this is not frequent and immunosuppressant therapy did not seem to affect the course of leprosy manifestations4,55. The course of leprosy seems not to be affected by immunosuppression55.
RENAL INVOLVEMENT: Renal involvement in leprosy was first described in the beginning of the XX century, through necropsy studies, in which glomerulonephritis and tubulointerstitial lesions were described28,36. Different renal lesions have been described in leprosy, including acute and chronic glomerulonephritis, interstitial nephritis, secondary amyloidosis and pyelonephritis19,41,48. There are several reports of renal involvement in leprosy, as summarized in Table 1.
Table 1. Studies and case reports on kidney involvement in leprosy.
| Reference | Number of cases | Age (years) | Gender | Clinical picture | Kidney biopsy |
|---|---|---|---|---|---|
| Iveson (1975)23 | 1 | 19 | M | Poliarthritis | Diffuse proliferative lesion |
| AKI | |||||
| Date (1977)12 | 19 | Proteinuria | Diffuse proliferative lesion | ||
| Hematuria | Amyloidosis | ||||
| Singhal (1977)53 | 3 | AKI | Acute tubular necrosis | ||
| Crescentic nephropathy | |||||
| Gupta (1981)20 | 21 | Diffuse proliferative lesion | |||
| Amyloidosis | |||||
| Phadnis (1982)42 | 50 | Membranoproliferative nephropathy | |||
| Membranous nephropathy | |||||
| Amyloidosis | |||||
| Chugh (1983)7 | 60 | Proteinuria | Mesangial proliferative lesion (8.3%) | ||
| Hematuria | Diffuse proliferative lesion (8.3%) | ||||
| AKI | Amyloidosis (5%) | ||||
| Jayalakshmi (1987)25 | 35 | 74 | AKI | Interstitial nephritis | |
| Amyloidosis | |||||
| Al-Mohaya (1988)2 | 1 | 17 | M | Proteinuria | Membranoproliferative nephropathy |
| Madiwale (1994)34 | 2 | 30-45 | M | Proteinuria | Crescentic nephropathy |
| Hematuria | |||||
| Ahsan (1995)1 | 1 | 79 | M | Hematuria | Diffuse proliferative lesion |
| AKI | |||||
| Lau (1995)31 | 1 | 71 | M | AKI | Interstitial nephritis |
| Drug hepatitis | |||||
| Nakayama (2001)38 | 199 | 47-74 | M (79.3%) | Amyloidosis (31%) | |
| Diffuse proliferative lesion (5%); | |||||
| Focal proliferative (4%); | |||||
| Membranoproliferative (2%); | |||||
| Membranous (1%); | |||||
| Mesangial proliferative lesion (0.5%) | |||||
| Glomerular sclerosis (11%) | |||||
| Tubulo-interstitial nephritis (9%) | |||||
| Granulomata (1%) | |||||
| Oliveira (2008)40 | 59 | 43 ± 15 | M (51%) | Concentration defect (84%) | No |
| Acidification defect (30%) | |||||
| Function loss (50%) | |||||
| Sharma (2010)49 | 1 | 25 | F | AKI | Crescentic nephropathy |
| Proteinuria | |||||
| Silva Junior (2010)52 | 1 | 58 | M | CKD | AA Amyloidosis |
| Daher (2011)10 | 923 | 41 ± 19 | M (53.3%) | Proteinuria (4.8%) | No |
| Hematuria (6.8%) | |||||
| Function loss (3.8%) |
M: Male; AKI: Acute kidney injury; CKD: Chronic kidney disease.
The exact mechanism that leads to glomerulonephritis in leprosy is not completely understood. The M. leprae may be directly involved in renal injury and it has already been detected in glomeruli of infected patients. The glomerular lesion is probably caused by immunologic mechanism, with complement decrease and immune complexes deposition in glomerular basement membrane, subendothelial and subepithelial space, detected by electronic microscopy19,41,48. Some studies have also detected mesangial proliferation and the presence of IgA in the mesangial area53. The pathophysiology of renal involvement in leprosy is illustrated in Fig. 1.
Fig. 1. Pathophysiology of renal involvement in leprosy. AKI = acute kidney injury; CKD = chronic kidney disease.
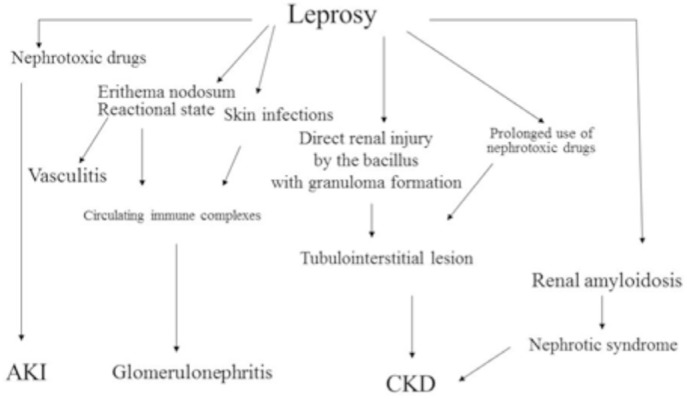
A consistent relation between the lepromatous form, erythema nodosum and kidney disease has been described in some studies18. Although leprosy nephropathy is more frequent in the multibacillary form, it can also occur in other forms and in the absence of the reactional state19.
A large retrospective study with 923 leprosy patients followed in a tertiary hospital in Brazil found acute kidney injury in 3.8% of cases, and 65% of them had the multibacillary form. Risk factors for kidney injury were reactional state, multibacillary classification and advanced age10.
RENAL LESION MECHANISM: Erythema nodosum leprosum is a reactional state characterized by immune complexes formation in circulation and subsequent deposition in vessels and tissues. Sometimes they are determined by Hansen's bacilli antigens which are released into circulation after the beginning of antibiotic therapy9. The antigens are recognized by host antibodies, and then immune complexes are formed. After this, immune complexes can deposit in the glomerulus or can occur by the formation of immune complexes in situ. However, not all glomerulonephritis in leprosy are associated with erythema nodosum, which raises the hypothesis of multifactorial influence in the development of leprosy nephropathy. In the virchowian form there is a cellular immunity decrease and a hyperactivation of humoral immunity, which makes the patient susceptible to the formation of immune complexes30.
The antigen that can induce the formation of immune complexes can originate from Hansen's bacilli or even from therapeutic agents. Anti-dapsone antibodies have been detected in the circulation of leprosy patients. Auto-antibodies have also been described in leprosy, mainly cryoglobulins with IgG and IgM13.
Some patients with lower limb ulcers and secondary infections by Streptococcus presented a higher frequency of glomerulonephritis7.
URINARY FINDINGS: Hematuria has been described in leprosy, mainly in the virchowian form and during erythema nodosum state, even in the absence of evident glomerulonephritis18. Microscopic hematuria is found in 12-16% of cases, which is higher than what is found in the general population (0.5-2%)7,17,57. This complication can disappear after a few months of specific treatment9.
Proteinuria has been described in several studies and its prevalence varies from 2.1 to 68%, and it is also more frequent in the multibacillary forms7,27,29,39,50. Proteinuria varies from 0.4 to 8.9g/day. Nephrotic syndrome is not frequent in leprosy. RAMANUJAM et al. 44 reported five cases in the virchowian form, four were in reactional state and only two had amyloid deposits detected.
Other urinary abnormalities, such as cylindruria and leukocyturia, are more frequently found in the virchowian form with reactional state. In the milder forms these abnormalities are uncommon44.
GLOMERULONEPHRITIS: Glomerulonephritis represents the most frequent type of kidney disease in leprosy. In renal biopsy studies glomerulonephritis was found in more than 30% of patients30, which is higher than what is found in necropsy studies (7%)13. In the multibacillary form, the prevalence of glomerulonephritis is higher8. Erythema nodosum has a strong correlation with the occurrence of glomerulonephritis, although there are some reports of its occurrence in reactional state type I7,9. Almost all kinds of glomerulonephritis have been described in leprosy7,13, and there is no specific histopathological pattern in leprosy nephropathy. There is a discrete predominance of membranoproliferative glomerulonephritis, which are in general associated with infectious disease-associated nephropathies20,24,42,49.
HISTOPATHOLOGICAL FINDINGS: The diversity of histopathological lesions found in leprosy suggests a heterogeneous disease but not necessarily with different etiologies13. Immunohistochemical studies with renal tissue have identified the presence of granular deposits of IgG and C3, and less frequently IgA, IgM and fibrin in the mesangium and in the glomerular capillaries, which is characterized by immune complex deposits or in situ formation. Electronic microscopy confirms the presence of dense granular deposits in mesangial-subendothelial and subepithelial regions14,26. Complement consumption in some cases reinforces the hypothesis of an immune complex-mediated disease30.
A study by GROVER et al. 19, with 72 leprosy patients undergoing renal biopsy found the following histopathological patterns: membranous nephropathy (31.5%) and mesangioproliferative glomerulonephritis (11.1%). VALLÉS et al. 56 reported one case of IgA nephropathy in a patient with the virchowian form, with reduction in glomerular filtration rate.
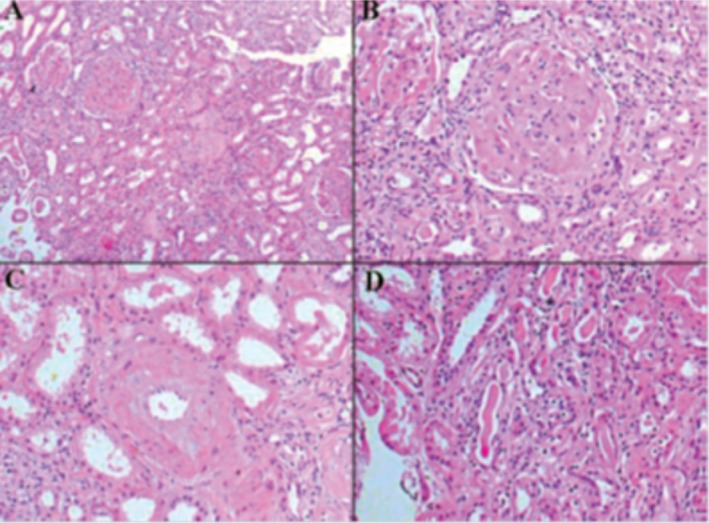
Several renal biopsy studies have been performed in leprosy. JOHNY et al. 26 performed renal biopsies in 35 patients with leprosy and identified histological abnormalities in 45% of them, and the most frequent was proliferative glomerulonephritis. GUPTA et al. 20 performed renal biopsies in 21 patients with virchowian leprosy, and found proliferative glomerulonephritis in 13 of them. GROVER et al. 19, in a study with 54 renal biopsies found 12 cases (22.2%) of diffuse proliferative glomerulonephritis (11 virchowian and one tuberculoid). They also found two cases of rapidly progressive glomerulonephritis, with acute kidney injury. Membranous nephropathy was found in 17 cases (31.5%). PHADNIS et al. 42 performed 50 renal biopsies and identified membranous nephropathy in two cases and membranoproliferative glomerulonephritis in six cases, of whom 45 had the lepromatous form and had reactional state. Interstitial nephritis was observed in 10 patients and amyloidosis in one case. Chronic kidney disease caused by secondary amyloidosis has also been described in leprosy52 (Fig. 2).
Fig. 2. Kidney biopsy from a patient with leprosy and chronic kidney disease showing amyloid deposits (A), H&E, x200; glomeruli without mesangial proliferation, with amyloid deposit in mesangium, H&E, x400; amyloid deposit, H&E x200; tubules without abnormalities, H&E x200. Reprinted from Silva Junior et al. Rev Soc Bras Med Trop. 2010;43:474-6.52 .
TUBULOINTERSTITIAL LESION: Interstitial nephritis is one of the most common histological findings in leprosy12,20,37. This has been described in patients with lepromatous leprosy, and is present in more than 20% of cases19. It seems to be related to disease duration and the long-term treatment with nephrotoxic drugs26.
The identification of specific lesions in leprosy is described as the presence of granulomas in renal interstitium, with evidence of mononuclear cells with vacuolized cytoplasm, without the presence of Hansen's bacilli43,47. Epithelioid granuloma and the Hansen's bacilli have already been detected in renal parenchyma38. The low incidence of granulomas in renal tissue is due to the fact that renal tissue presents a resistance to M. leprae or the fact that the bacteria has a low affinity to renal tissue42.
The occurrence of tubular dysfunction is frequent, varying from 25 to 85% of cases, in both multibacillary and paucibacillary forms7,40. Urinary acidification defect has been described in 20 to 32% of patients, and urinary concentration defect in 85% of cases16,40. Renal tubular acidosis has also been described16,21,40.
CHRONIC KIDNEY DISEASE: Chronic kidney disease (CKD) has been reported as one of the causes of death in leprosy, mainly in the first studies of leprosy nephropathy5,28,36,43,. CKD is mainly caused by amyloidosis and is also more frequent in the virchowian form26,33,52. It has also been reported in patients with the tuberculoid form33. A correlation between the duration of the disease and the development of amyloidosis has not been observed26. A positive correlation was detected between the occurrence of erythema nodosum and secondary amyloidosis in leprosy15,32,33. Serum levels of amyloid protein A increases in erythema nodosum episodes and remains high for several months. LOMONTE et al. 32 described the evolution of eight patients with leprosy who developed CKD and required renal replacement therapy.
DRUGS TOXICITY: Despite not being common, renal abnormalities due to leprosy specific treatment have been described. There are reports on acute tubular necrosis, interstitial nephritis and papillary necrosis causing acute kidney injury in leprosy7,15.
Acute kidney injury can occur due to interstitial nephritis secondary to rifampicin use, which is more common with higher doses (900-1200mg) than the usual (450-600mg)22. Dapsone can induce hemolysis and intravascular coagulation, which can lead to acute tubular necrosis54.
TREATMENT: Leprosy treatment encompasses specific therapy to overturn M. leprae, avoid immunological complications and prevent physical deformities, simultaneously promoting physical and psychosocial rehabilitation. Additionally, health authority notification is mandatory35. WHO-standardized leprosy therapy includes rifampicin, dapsone and clofazimine. Prednisone (1 to 2 mg/kg/day) and non-steroidal anti-inflammatory drugs (NSAI) may be used to control acute immunological episodes. Erythema nodosum leprosum may sometimes have a protracted course (months, or years) and is usually treated with NSAI, steroids, thalidomide, clofazimine and pentoxiphyline. It must be kept in mind that all are potentially nephrotoxic. Hemodialysis or kidney transplant are alternatives in treating leprosy ESRD. Post-transplant immunosuppression apparently does not modify leprosy response to drugs. However, acute transitory deterioration of its course has been reported4.
CONCLUSION
Renal involvement is an important complication in leprosy, which should be investigated in every patient. Multibacillary status seems to be the main risk factor for kidney dysfunction in this disease. Different kinds of glomerulopathy have been described in association with leprosy. Specific treatment seems to impact on renal function improvement.
Footnotes
Financial Support: This research was supported by the Brazilian Research Council (Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, Brazil, Protocol 475040/2011-2).
REFERENCES
- 1.Ahsan N, Wheeler DE, Palmer BF. Leprosy-associated renal disease: case report and review of the literature. J Am Soc Nephrol. 1995;5:1546–52. doi: 10.1681/ASN.V581546. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mohaya SA, Coode PE, Alkhder AA, Al-Suhaibani MO. Renal granuloma and mesangial proliferative glomerulonephritis in leprosy. Int J Lepr Other Mycobact Dis. 1988;56:599–602. [PubMed] [Google Scholar]
- 3.Araújo MG. Hanseníase no Brasil. Rev Soc Bras Med Trop. 2003;36:373–82. [PubMed] [Google Scholar]
- 4.Ardalan M, Ghaffari A, Ghabili K, Shoja MM. Lepromatous leprosy in a kidney transplant recipient: a case report. Exp Clin Transplant. 2011;9:203–6. [PubMed] [Google Scholar]
- 5.Bernard JC, Vazquez CAJ. Visceral lesions in lepromatous leprosy. Study of sixty necropsies. Int J Lepr Other Mycobact Dis. 1973;41:94–101. [PubMed] [Google Scholar]
- 6.Bhat RM, Prakash C. Leprosy: an overview of pathophysiology. Interdiscip Perspect Infect Dis. 2012;2012:181089. doi: 10.1155/2012/181089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chugh KS, Damie PB, Kaur S, Shama BK, Kumar B, Sakhuja V, et al. Renal lesions in leprosy amongst north Indian patients. Postgrad Med J. 1983;59:707–11. doi: 10.1136/pgmj.59.697.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chugh KS, Sakhuja V. End stage renal disease in leprosy. Int J Artif Organs. 1986;9:9–10. [PubMed] [Google Scholar]
- 9.Cologlu AS. Immune complex glomerulonephritis in leprosy. Lepr Rev. 1979;50:213–22. [PubMed] [Google Scholar]
- 10.Daher EF, Silva GB, Jr, Cezar LC, Lima RS, Gurjão NH, Mota RM, et al. Renal dysfunction in leprosy: a historical cohort of 923 patients in Brazil. Trop Doct. 2011;41:148–50. doi: 10.1258/td.2011.100436. [DOI] [PubMed] [Google Scholar]
- 11.Date A, Johny KV. Glomerular subepithelial deposits in lepromatous leprosy. Am J Trop Med Hyg. 1975;24:853–6. doi: 10.4269/ajtmh.1975.24.853. [DOI] [PubMed] [Google Scholar]
- 12.Date A, Thomas A, Mathai R, Johny KV. Glomerular pathology in leprosy. An electron microscopic study. Am J Trop Med Hyg. 1977;26:266–72. doi: 10.4269/ajtmh.1977.26.266. [DOI] [PubMed] [Google Scholar]
- 13.Date A. The immunological basis of glomerular disease in leprosy: a brief review. Int J Lepr Other Mycobact Dis. 1982;50:351–3. [PubMed] [Google Scholar]
- 14.Date A, Neela P, Shastry JC. Membranoproliferative glomerulonephritis in a tropical environment. Ann Trop Med Parasitol. 1983;77:279–85. doi: 10.1080/00034983.1983.11811708. [DOI] [PubMed] [Google Scholar]
- 15.Date A, Harihar S, Jeyavarthini SE. Renal lesions and other major findings in necropsies of 133 patients with leprosy. Int J Lepr Other Mycobact Dis. 1985;53:455–60. [PubMed] [Google Scholar]
- 16.Drutz DJ, Gutman RA. Renal tubular acidosis in leprosy. Ann Int Med. 1971;75:475–6. doi: 10.7326/0003-4819-75-3-475_2. [DOI] [PubMed] [Google Scholar]
- 17.Faria JBL. Significado da hematúria no diabetes mellitus. [Dissertação] São Paulo: Escola Paulista de Medicina, Curso de Pós-graduação em Nefrologia; 1986. [Google Scholar]
- 18.Gelber RH. Erythema nodosum leprosum associated with azotemic acute glomerulonephritis and recurent haematuria. Int J Lepr Other Mycobact Dis. 1986;54:125–7. [PubMed] [Google Scholar]
- 19.Grover S, Bobhate SK, Chaubey BS. Renal abnormalities in leprosy. Lepr India. 1983;55:286–91. [PubMed] [Google Scholar]
- 20.Gupta SC, Bajaj AK, Govil DC, Sinha SN, Kumar R. A study of percutaneous renal biopsy in lepromatous leprosy. Lepr India. 1981;53:179–84. [PubMed] [Google Scholar]
- 21.Gutman RA, Lu WH, Drutz DJ. Renal manifestations of leprosy: impaired acidification and concentration of urine in patients with leprosy. Am J Trop Med Hyg. 1973;22:223–8. doi: 10.4269/ajtmh.1973.22.223. [DOI] [PubMed] [Google Scholar]
- 22.Humes HD, Weimberg JM. Toxic nephropathies, acute alergic or hypersensitivity tubulointerstitial nephropathy. In: Brenner BM, Rector FC Jr, editors. The kidney. 3. ed. v. 2. Philadelphia: WB Saunders; 1986. p. 1515. [Google Scholar]
- 23.Iveson JM, McDougall AC, Leathem AJ, Harris HJ. Lepromatous leprosy presenting with polyarthritis, myositis, and immune-complex glomerulonephritis. Br Med J. 1975;3:619–21. doi: 10.1136/bmj.3.5984.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain PK, Kumar S, Govil DC, Mittal VP, Agarwal N, Arora RC, et al. In: X International Congress of Nephrology. London: 1987. Renal changes in leprosy and its reactions; p. 69. Abstracts. [Google Scholar]
- 25.Jayalakshmi P, Looi LM, Lim KJ, Rajogopalan K. Autopsy findings in 35 cases of leprosy in Malaysia. Int J Lepr Other Mycobact Dis. 1987;55:510–4. [PubMed] [Google Scholar]
- 26.Johny KV, Karat ABA, Rao PPS, Date A. Glomerulonephritis in leprosy: a percutaneous renal biopsy study. Lepr Rev. 1975;46:29–37. doi: 10.5935/0305-7518.19750005. [DOI] [PubMed] [Google Scholar]
- 27.Kanwar AJ, Bharija SC, Belhaj MS. Renal functional status in leprosy. Indian J Lepr. 1984;56:595–9. [PubMed] [Google Scholar]
- 28.Kean B, Childress ME. A summary of 103 autopsies on leprosy patients on the Isthmus of Panama. Int J Lepr. 1942;10:51–9. [Google Scholar]
- 29.Kirsztajn GM, Nishida SK, Silva MS, Ajzen H, Pereira AB. Renal abnormalities in leprosy. Nephron. 1993;65:381–4. doi: 10.1159/000187517. [DOI] [PubMed] [Google Scholar]
- 30.Kirsztajn GM, Pereira AB. Comprometimento renal na hanseníase. In: Cruz J, Barros RT, editors. Atualidades em nefrologia 4. São Paulo: Sarvier; 1994. pp. 144–53. [Google Scholar]
- 31.Lau G. A fatal case of drug-induced multi-organ damage in a patient with Hansen's disease: dapsone syndrome or rifampicin toxicity? Forensic Sci Int. 1995;73:109–15. doi: 10.1016/0379-0738(95)01719-y. [DOI] [PubMed] [Google Scholar]
- 32.Lomonte C, Chiarulli G, Cazzato F, Giammaria B, Marchio G, Losurdo N, et al. End stage renal disease in leprosy. J Nephrol. 2004;17:302–5. [PubMed] [Google Scholar]
- 33.MacAdam KP, Anders RS, Smith SR, Russel DA, Prince MA. Association of amyloidosis and erythema nodosum leprosum reactions and recurrent neutrophil leucocytosis in leprosy. Lancet. 1975;2((7935)):572–3. doi: 10.1016/s0140-6736(75)90168-3. [DOI] [PubMed] [Google Scholar]
- 34.Madiwale CV, Mittal BV, Dixit M, Acharya VN. Acute renal failure due to crescentic glomerulonephritis complicating leprosy. Nephrol Dial Transplant. 1994;9:178–9. [PubMed] [Google Scholar]
- 35.Ministério da Saúde . Secretaria de Vigilância em Saúde. Guia de vigilância epidemiológica. 7a ed. Brasília: Ministério da Saúde; 2009. [Google Scholar]
- 36.Mitsuda K, Ogawa M. A study of 150 autopsies on cases of leprosy. Int J Lepr. 1937;5:53–60. [Google Scholar]
- 37.Mittal MM, Maheshwari HB, Kumar S. Renal lesions in leprosy. Arch Pathol. 1972;93:8–12. [PubMed] [Google Scholar]
- 38.Nakayama EE, Ura S, Fleury RN, Soares V. Renal lesions in leprosy: a retrospective study of 199 autopsies. Am J Kidney Dis. 2001;38:26–30. doi: 10.1053/ajkd.2001.25177. [DOI] [PubMed] [Google Scholar]
- 39.Nigam P, Pant KC, Kapoor KK, Kumar A, Saxena SP, Sharma SP, et al. Histo-functional status of kidney in leprosy. Indian J Lepr. 1986;58:567–75. [PubMed] [Google Scholar]
- 40.Oliveira RA, Silva GB, Jr, Souza CJ, Vieira EF, Mota RM, Martins AM, et al. Evaluation of renal function in leprosy: a study of 59 consecutive patients. Nephrol Dial Transplant. 2008;23:256–62. doi: 10.1093/ndt/gfm568. [DOI] [PubMed] [Google Scholar]
- 41.Peter KS, Vijayakumar T, Vasudevan DM, Devi KR, Mathew MT, Gopinath T. Renal involvement in leprosy. Lepr India. 1981;53:163–78. [PubMed] [Google Scholar]
- 42.Phadnis MD, Mehta MC, Bharaswadker MS, Kolhatkar MK, Bulakh PN. Study of renal changes in leprosy. Int J Lepr Other Mycobact Dis. 1982;50:143–7. [PubMed] [Google Scholar]
- 43.Powell CS, Swan LL. Leprosy: pathologic changes observed in fifty consecutive necropsies. Am J Pathol. 1955;31:1131–47. [PMC free article] [PubMed] [Google Scholar]
- 44.Ramanujam MK, Ramu G, Balakhrishnan S, Desikan KV. Nephrotic syndrome complicating lepromatous leprosy. India J Med Res. 1973;61:548–56. [PubMed] [Google Scholar]
- 45.Renault CA, Ernst JD. Mycobacterium leprae . 7th ed. Mandell: Mandell, Douglas, and Bennett's principles and practice of infectious diseases; Philadelphia: Churchill Livingstone Elsevier; 2010. pp. 3165–76. [Google Scholar]
- 46.Rodrigues LC, Lockwood DNJ. Leprosy now: epidemiology, progress, challenges, and research gaps. Lancet Infect Dis. 2011;11:464–70. doi: 10.1016/S1473-3099(11)70006-8. [DOI] [PubMed] [Google Scholar]
- 47.Sainani GS, Rao KV. Renal changes in leprosy. J Assoc Physicians India. 1974;22:659–64. [PubMed] [Google Scholar]
- 48.Sengupta U, Ramu G, Sinha S, Ramanathan VD, Desikan KV. Immunoglobulins in the urine of leprosy patients. Int J Lepr Other Mycobact Dis. 1983;51:409–10. [PubMed] [Google Scholar]
- 49.Sharma A, Gupta R, Khaira A, Gupta A, Tiwari SC, Dinda AK. Renal involvement in leprosy: report of progression from diffuse proliferative to crescentic glomerulonephritis. Clin Exp Nephrol. 2010;14:268–71. doi: 10.1007/s10157-009-0255-6. [DOI] [PubMed] [Google Scholar]
- 50.Shwe T. Renal involvement in leprosy. Trans R Soc Trop Med Hyg. 1972;66:26–7. doi: 10.1016/0035-9203(72)90050-8. [DOI] [PubMed] [Google Scholar]
- 51.Silva GB, Junior, Daher EF. Renal involvement in leprosy: retrospective analysis of 461 cases in Brazil. Braz J Infect Dis. 2006;10:107–12. doi: 10.1590/s1413-86702006000200007. [DOI] [PubMed] [Google Scholar]
- 52.Silva GB, Junior, Barbosa OA, Barros RM, Carvalho P dos R, Mendoza TR, Barreto DM, et al. Amiloídose e insuficiência renal crônica terminal associada à hanseníase. Rev Soc Bras Med Trop. 2010;43:474–6. doi: 10.1590/s0037-86822010000400031. [DOI] [PubMed] [Google Scholar]
- 53.Singhal PC, Chugh KS, Kaur S, Malik AK. Acute renal failure in leprosy. Int J Lepr Other Mycobact Dis. 1977;45:171–4. [PubMed] [Google Scholar]
- 54.Thunga G, Sam KG, Patel D, Khera K, Sheshadhri S, Bahuleyan S, et al. Effectivenes of hemodialysis in acute dapsone overdose: a case report. Am J Emerg Med. 2008;26:1070. doi: 10.1016/j.ajem.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 55.Trindade MA, Palermo ML, Pagliari C, Valente N, Naafs B, Massarollo PC, et al. Leprosy in transplant recipients: report of a case after liver transplantation and review of the literature. Transpl Infect Dis. 2011;13:63–9. doi: 10.1111/j.1399-3062.2010.00549.x. [DOI] [PubMed] [Google Scholar]
- 56.Vallés M, Cantarelli C, Fort J, Carrera M. IgA nephropathy in leprosy. Arch Intern Med. 1982;142:1238. doi: 10.1001/archinte.1982.00340190198034. [DOI] [PubMed] [Google Scholar]
- 57.Vehaskari VM, Rapola J, Koskimies O, Savilahti E, Vilska J, Hallman N. Microscopic hematuria in school-children: epidemiology and clinicopathologic evaluation. J Pediatr. 1979;95((5 Pt 1)):676–84. doi: 10.1016/s0022-3476(79)80710-6. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization Global leprosy situation, 2010. Wkly Epidemiol Rec. 2010;85:337–48. [PubMed] [Google Scholar]


