Abstract
Purpose
The anticancer property and cytoprotective role of selenium in chemotherapy have been reported. However, the combination effects of selenium on chemotherapy for advanced breast cancer have not yet been clearly defined. The purpose of this study was to investigate the combined effects of selenium on chemotherapy using docetaxel on breast cancer cell lines.
Methods
Under adherent culture conditions, two breast cancer cell lines, MDA-MB-231 and MCF-7, were treated with docetaxel at 500pM and selenium at 100nM, 1µM, or 10µM. Changes in cell growth, cell cycle duration, and degree of apoptosis after 72 hours in each treated group were evaluated.
Results
In the MDA-MB-231 cells, the combination therapy group (docetaxel at 500pM plus selenium at 10µM) showed a significantly decreased percentage of cell growth (15% vs. 28%; P = 0.004), a significantly increased percentage of late apoptosis (63% vs. 26%; P = 0.001), and an increased cell cycle arrest in the G2/M phase (P = 0.001) compared with the solitary docetaxel therapy group. Isobologram analysis demonstrated the synergistic effect of the combination therapy in the MDA-MB-231 cells. However, in the MCF-7 cells, no significant differences in the percentage of cell growth apoptosis, the percentage of apoptosis, and the pattern of cell cycle arrest were noted between the combination therapy groups and the solitary docetaxel therapy group.
Conclusion
Our in vitro study indicated that the combination of selenium with docetaxel inhibits cell proliferation through apoptosis and cell arrest in the G2/M phase in MDA-MB-231 breast cancer cells.
Keywords: Breast neoplasms, Selenium, Docetaxel, Combination drug therapy
INTRODUCTION
Breast cancer is the most common invasive cancer and is the second most common cause of deaths in women. Breast cancer is usually treated surgically, followed by chemotherapy and immunotherapy or radiation therapy, or both in cases of advanced cancer [1,2]. Metastatic breast cancer is the most serious and incurable disease with a 2- to 3-year median overall survival [3]. In advanced breast cancer patients, chemotherapy is generally recommended in patients with hormone receptor negative tumors, endocrine-resistant disease of the luminal subtype, and rapidly proliferating disease [4,5]. Generally, cyclophosphamide and adriamycin are used for chemotherapy, and sometimes, a taxane drug such as docetaxel is added [5,6].
Docetaxel is an antimitotic chemotherapeutic agent used for treating patients, who have locally advanced or metastatic breast cancer and have undergone unsuccessful anthracycline-based chemotherapy [5,6,7]. Docetaxel exerts its cytotoxicity by promoting tubulin polymerization and stabilizing microtubule assembly, while preventing physiological microtubule depolymerization [4]. This action significantly decreases free tubulin needed for microtubular formation and inhibits mitotic cell division between metaphase and anaphase, thus preventing further cell progeny. Clinically, docetaxel may inhibit the proliferation of breast cancer cells, but the drug results in several adverse effects in normal cells [8].
Selenium is well known as an essential micronutrient for human health [9] and has been recently regarded as a potent nutritional anticancer element. In breast cancer, various ecological studies and case-control studies have found an inverse relationship between selenium levels and cancer risk and mortality [10,11]. An experimental study showed that the administration of selenium reduced the rate of breast cancer cell growth in an in vitro setting [12]. Selenium administration of 0.8-µg/g body weight was associated with an 80%-93% reduction in the rate of breast cancer cell growth, without apparent adverse effects on the host [12].
Some cancer patients experience several adverse effects after radiotherapy and chemotherapy as well as develop chronic lymphedema resulting from cellular processes during oxidative normal cell damage in the human body [13]. Selenium has recently been suggested as an alternative remedy against chemotherapy and radiotherapy-associated side effects as well as its effects on lymphedema [14,15,16,17,18,19].
Selenium supplementation may be beneficial in advanced breast cancer patients undergoing chemotherapy, considering its anticancer properties and added potential to control side effects and lymphoedema caused by chemotherapy. However, the combination effects of selenium in chemotherapy for advanced breast cancer have not yet been clearly defined.
Therefore, we hypothesized that a combination therapy with selenium may be beneficial for inhibiting breast cancer cell growth in an experimental setting of conventional chemotherapy with docetaxel for advanced breast cancer. The aim of this study was to investigate the combination effects of selenium in chemotherapy with docetaxel in breast cancer cells, MDA-MB-231 and MCF-7, in comparison with solitary chemotherapy therapy.
METHODS
Cell culture
Two human breast cancer cell lines (MDA-MB-231 and MCF-7) were obtained from American Type Cell Culture (ATCC, Bethesda, MD, USA). The experimental cells were cultured in tissue culture dishes (Cat no: 353003, Falcon, San Jose, CA, USA) in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). Cultured cells (5 × 105) were grown at 37℃ in a humidified atmosphere containing 5% CO2.
Assessment of cell growth after treatment
For each cell line, 5 × 105 cells were seeded in DMEM containing 10% FBS. After 24 hours, the cells were washed with phosphate buffered saline (PBS) and cultured in fresh media. First, 500pM was determined as a therapeutic docetaxel dose for the MDA-MB-231 and MCF-7 cells for 72 hours. Next, selenium at 100nM, 1µM, or 10µM was added to the MDA-MB-231 and MCF-7 cells for 72 hours to confirm the independent effects of selenium on cell proliferation and survival at different concentrations before we evaluated the effect of selenium combination therapy. Second, docetaxel alone at 500pM (DT 500pM), docetaxel at 500pM plus selenium at 100nM, 1µM or 10µM (DT 500pM + SN 100nM; DT 500pM + SN 1µM; DT 500pM + SN 10µM) were added to the MDA-MB-231 and MCF-7 cells for 72 hours. The cells were then quantified in a Neubauer chamber using a hemocytometer after incubating with 0.4% trypan blue dye. All measurements were performed in triplicate, and each experiment was repeated at least three times.
Assessment of combined drug effects using isobologram analysis
Traditionally, isobologram analysis has been used as a method for identifying the combined effect of multiple drugs in terms of the additive, synergistic, or antagonistic effect. Because the combination of selenium and docetaxel was used in a nonconstant dose ratio, a normalized isobologram for the two drugs at their ED50 was constructed automatically using CompuSyn ver. 1.0 (ComboSyn Inc., Paramus, NJ, USA). An additive effect (if points fall on the hypotenuse), synergism (if points fall in the lower left), or antagonism (if points fall in the upper right) is indicated according to the positions of the combination data points in the normalized isobologram. The combination index (CI) provides a means to analyze the combined effects using a median-effect plot analysis. CI values <1.0, =1.0, and >1.0 indicate synergistic, additive, and antagonistic effects, respectively.
Apoptosis analysis using Annexin V staining
Annexin V staining was performed according to the manufacturer's protocol (Pharmingen, BD Biosciences, San Jose, CA, USA). Trypsin-ethylenediaminetetraacetic acid was used to obtain single-cell suspensions. After centrifuging treated cell lines, the cells were washed twice with cold PBS and resuspended in a binding buffer (10mM HEPES, pH 7.4, 150mM NaCl, 5mM KCl, 1mM MgCl2, and 1.8mM CaCl2) at a concentration of 1 × 106 cells/mL. An aliquot (100 µL) of the solution containing 1 × 105 cells was transferred to a 5-mL culture tube, and 5-µL Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) were added to each tube. After vortexing the tubes, cells were incubated for 15 minutes at room temperature (25℃) in the dark and 400 µL of the binding buffer was added to each tube. Flow cytometry was performed with a FACSCalibur system (BD Biosciences) within 1 hour.
Cell cycle analysis
The growth of both cancer cell lines was inhibited probably because their cell cycles were arrested, and we determined whether the growth inhibition was a result of cell cycle arrest by analyzing the proportions of cells in different cell cycle phases. After 72 hours of treatment in each group, the cells were harvested, fixed in 70% ethanol for 1 hour, and washed with PBS. Next, the cells were treated with 100 µg/mL of RNase A for 1 hour at 37℃ and were stained with 10 µg/mL of PI. Flow cytometry was performed in triplicate for each experiment using a FACSCalibur system.
Statistical analysis
All the data were compiled from a minimum of three replicate experiments. Student t-tests were used to compare results of treated and control cells. To compare the normally distributed continuous variables, one-way analysis of variance tests were used. To compare the nonnormally distributed continuous variables, Kruskal-Wallis tests were used. Chi-square tests were used to compare the nominal variables. Data were analyzed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Two-sided P-values less than 0.05 were considered statistically significant.
RESULTS
Cell growth after combined treatment with docetaxel and selenium in MDA-MB-231 and MCF-7 cell lines
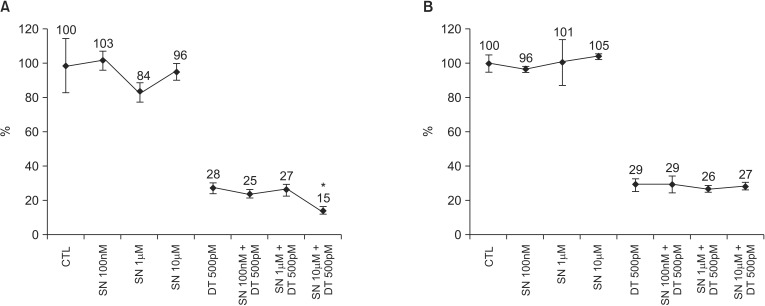
In the solitary selenium therapy model, there were no significant differences in cell growth among the control cells and cells treated with selenium at 100nM, 1µM, or 10µM in MDA-MB-231 cell lines (P = 0.824) and MCF-7 cell lines (P = 0.453) (Fig. 1). In the DT 500pM + SN combination therapy model, cell growth decreased significantly in the group with a DT 500pM + SN 10µM compared with the DT 500pM (15% vs. 28%, P = 0.004) in the MDA-MB 231 cell line (Fig. 1). However, cell growth did not significantly decrease in all DT 500pM + SN combination therapy groups compared with the solitary therapy group in MCF-7 cell lines (Fig. 1).
Fig. 1.
Cell growth after combined treatment of docetaxel at 500pM and selenium at 100nM, 1µM, or 10µM in the MDA-MB-231 cell line (A) and the MCF-7 cell line (B). Cells were stained with trypan blue to determine cell viability and were quantified using a hemocytometer. Relative cell growth rates are shown as percent survival versus indicated cells after treatment with the drug at different concentrations. The data represent the means of at least three independent experiments and the corresponding standard errors. *P-value less than 0.05.
Combined effect of docetaxel and selenium in MDA-MB-231 and MCF-7 cells
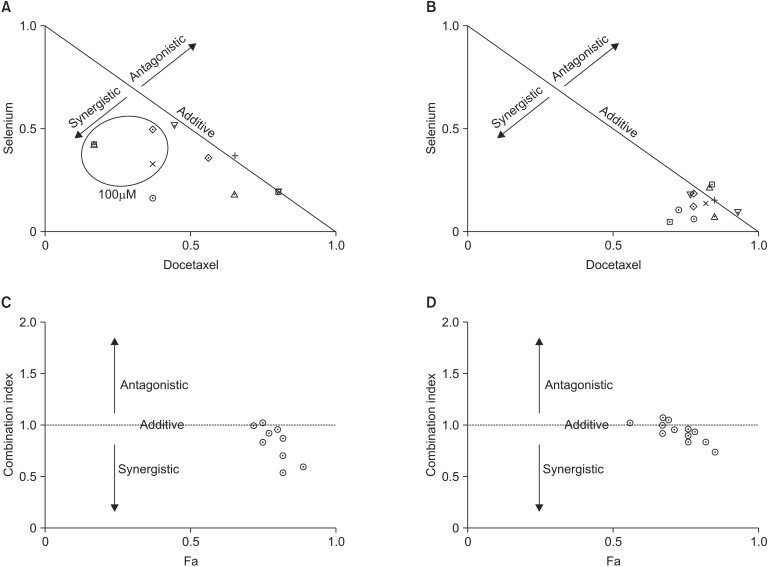
Normalized isobolograms were constructed for MDA-MB-231 and MCF-7 cells using dose-response curves. In MDA-MB-231 cells, synergism was generally identified when the cells were exposed to 100µM of selenium with docetaxel (Fig. 2). And, an additive effect was generally identified when the cells were exposed to other doses of selenium with docetaxel. In MCF-7 cells, an additive effect was generally identified when the cells were exposed to selenium with docetaxel (Fig. 2).
Fig. 2.
Normalized isobologram for selenium and docetaxel in the MDA-MB-231 (A) and MCF-7 (B) cell lines. Diagonal experimental combination data points, represented by dots located lower left, on the diagonal line, or upper right, indicate synergism, additivity, and antagonism, respectively. Fractional affected-combination index (Fa-CI) plot in combination index analysis in the MDA-MB-231 (C) and MCF-7 (D) cell lines. Combination index values <1, =1, or >1 indicate synergism, additivity, and antagonism, respectively.
Apoptosis and cell cycle analysis after combined treatment with docetaxel and selenium in the MDA-MB-231 and MCF-7 cell lines
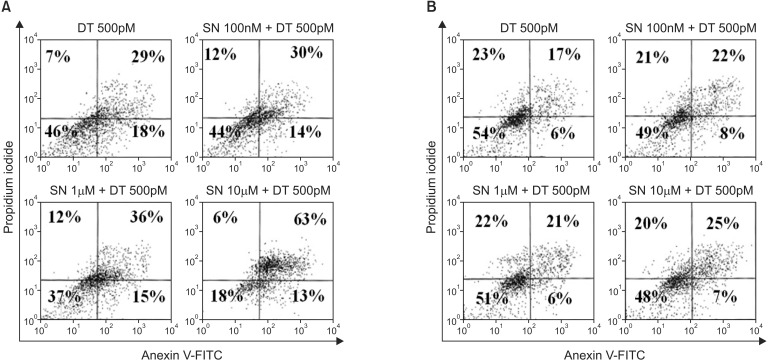
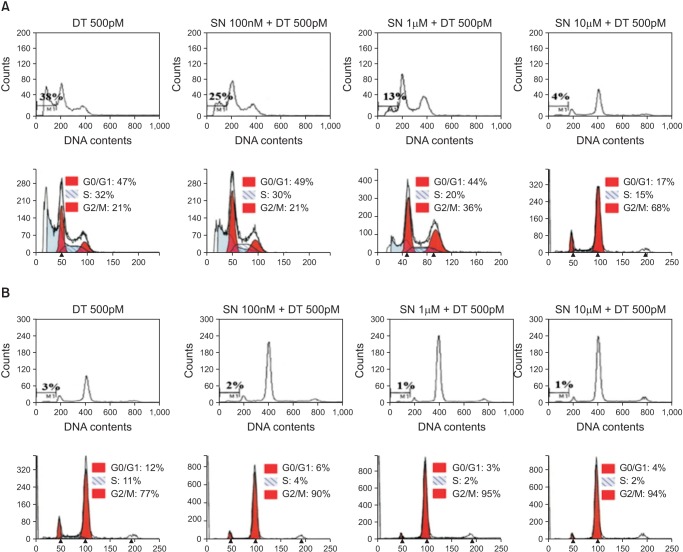
In the MDA-MB-231 cell lines, early apoptosis in all combined therapy groups was not significantly different from the DT 500pM group (13%-15% vs. 18%, P = 0.125). However, late apoptosis increased in the DT 500pM + SN 10µM compared with the DT 500pM and other combination therapy groups (63% vs. 29%-36%) (Fig. 3). Cell cycle analysis indicated a dominant increase in cell cycle arrest in the G2/M phase in the DT 500pM + SN 10µM groups compared with the other treatment groups (Fig. 4).
Fig. 3.
Apoptosis after combined treatment with docetaxel at 500pM and selenium at 100nM, 1µM, or 10µM in the MDA-MB-231 cell line (A) and the MCF-7 cell line (B). All cells were stained with fluorescein isothiocyanate (FITC) conjugated Annexin V in a buffer containing propidium iodide and were analyzed using flow cytometry. For each treatment, the percentage of viable cells is shown in the lower left quadrant, for which both Annexin V and propidium iodide levels are low. Data from a representative experiment (from a total of three) are shown.
Fig. 4.
Cell cycle analysis after combined treatment with docetaxel at 500pM and selenium at 100nM, 1µM, or 10µM in the MDA-MB-231 cell line (A) and the MCF-7 cell line (B). The percentage of cells at each stage of the cell cycle was analyzed using flow cytometry after DNA staining with propidium iodide. Data from a representative experiment (from a total of three) are shown.
In MCF-7 cell lines, the amounts of early apoptosis (6%-8 % vs. 6%) and late apoptosis (21%-25% vs. 17%) were not significantly different in all the DT 500pM + SN combination therapy groups compared with the DT 500pM group (Fig. 3). Cell cycle analysis indicated a small increase in cell cycle arrest in the G2/M phase in the DT 500pM + SN combination therapy groups compared with the DT 500pM group, but no remarkable difference in cell cycle arrest in the G2/M phase was observed among the DT 500pM + SN combination therapy groups (Fig. 4).
DISCUSSION
In this experimental study, we investigated the combined effect of selenium and docetaxel-based chemotherapy on the growth suppression of two breast cancer cell lines (MDA-MB-231 and MCF-7). The results of our study showed that the synergistic effect of selenium on chemotherapy with docetaxel for advanced breast cancer cells was cell line-specific and selenium dose-specific. Compared with solitary docetaxel therapy, only the MDA-MB-231 cell lines treated with DT 500pM + SN 10µM showed a higher suppression of cancer cell growth. Additionally, we found increased percentages of apoptotic cells and increased DNA contents in the G2/M phase in the MDA-MB-231 cell lines treated with DT 500pM + SN 10µM, and this result was consistent with the cell growth results. However, in our study, we could not observe any synergistic effect of selenium on docetaxel therapy in the MCF-7 cell line.
Clinically, selenium may be an attractive natural additive for chemotherapy for advanced breast cancer, which is not easily curable. Selenium is involved in antioxidant reactions and redox regulation pathway as a component of seleno-proteins and as a source of seleno-metabolites [20]. Numerous experimental and clinical studies have demonstrated that selenium supplementation reduces the incidence of human cancers, including breast, prostate, lung, colon, and liver cancers [21,22,23]. Selenium has been established as a crucial cancer preventive element and is thought to reduce cancer progression. Although there have been no definite clinical studies, some laboratory studies have shown that selenium inhibits breast cancer cell growth in a dose-dependent manner.
Multiple chemotherapeutic agents have been developed to treat patients with advanced breast cancer. Docetaxel is generally accepted as the chemotherapy agent for patients with advanced breast cancer in whom prior chemotherapy has failed [5], but its clinical efficacy can be compromised by serious docetaxelrelated side effects such as serious neutropenia, fluid retention, hair loss, nail changes, and hypersensitivity reactions [8]. Controlling side effects associated with docetaxel therapy may improve quality of life and clinical outcomes in patients with chemotherapy. Adverse effects of chemotherapy are associated with an increase in free radicals and related oxidative damage in normal human cells [24]. Recently, selenium compounds have been suggested as toxicity antagonists that prevent side effects related to chemotherapy. Some animal studies have suggested that sodium selenite may reduce the side effects of chemotherapy, such as cisplatin or Adriamycin [15,17,18]. Patients often suffer from secondary lymphedema after surgical and radiological therapy because of fluid retention due to surgical- or radiation-related damage to the lymphatic system [13]. Selenium may improve redox balance in sparsely perfused edematous tissue and hence, may be a useful agent in controlling and alleviating lymphedema [25].
Considering that selenium can inhibit cancer cell growth in breast cancer patients, and can reduce the side effects of chemotherapy and lymphedema, consensus about the use of combination therapy using selenium may be a benefit treatment option in these patients. We first identified the independent effect of selenium on cancer cell growth in stepwise dose increments before evaluating the effects of selenium combination therapy. This experiment therefore precludes bias and reduces confusion regarding efficacy of selenium plus docetaxel combination therapy. It clearly verifies the efficacy of selenium as an additive to the docetaxel chemotherapy in a dose-dependent manner. Considering that only 10µM of selenium can significantly reduce the cell growth in MDA-MB-231 cell lines, the selenium combined effect is considered dose-dependent and is targeted to specific cell lines, that is, MDA-MB cell lines. Apoptosis analysis using the Annexin V-FITC staining showed high concentrated apoptosis of cells in 10µM selenium combination therapy, which inhibited MDA-MB-231 cells significantly. These results are consistent with our cell analysis data that demonstrated increased arrest in the G2/M cycle in the combination therapy group.
We could not demonstrate the exact mechanism underlying the selenium combined with docetaxel chemotherapy in this study. The mechanisms by which selenium might act on the cancer progression are not well known; however, apoptosis and programmed cell death have been postulated as primary mechanism for inhibiting cancer growth by selenium compounds [26]. Few studies have shown that selenium inhibited the growth of human breast cancer cells by mitochondria-mediated apoptosis and arresting the G1 cell cycle [20,27]. Selenium-induced G1-phase arrest was associated with decreased expression levels of cyclin D1, cyclin D3, CDK4, and CKD6 and increased expression levels of p15 INK4B, p21 Waf1/Cip1, and p53 [28].
An interesting finding of the study is that only estrogen receptor-negative MDA-MB-231 cells showed a significant synergistic effect of selenium with the docetaxel combination therapy. Most breast cancer patients are female, and estrogen is strongly associated with breast cancer cell growth. Estrogen is thought to be responsible for the regulation of the expression of selenium-binding protein, which induces the inhibition of cancer cell proliferation during selenium treatment [29]. In previous in vivo and in vitro studies, selenium induced greater cell death and apoptosis in estrogen receptor-positive MCF-7 cells but not in estrogen-negative MDA-MB-231 cells [29,30]. The mechanism of growth inhibition in estrogen receptor-positive MCF-7 cells is thought to occur through disruption of estrogen signaling [30]. However, selenium seemed to act differently on cancer cells when combined with docetaxel in the combined chemotherapy model. In the combined model of docetaxel chemotherapy with selenium, a synergistic effect of selenium on cell death was not observed in estrogen receptor-positive MCF-7 cells but was observed in estrogen-negative MDA-MB-231 cells. The mechanism responsible for cell death through disruption of estrogen signaling does not seem to explain our study results. The cytotoxic effect of docetaxel occurs mainly through blocking mitosis by inhibiting mitotic spindle assembly, and selenium may help induce this antimitosis reaction in MDA-MB-231 cells when given in combination with docetaxel instead of through the direct inhibition of cell growth by itself. However, it is unclear why the synergistic effect of selenium in combined docetaxel therapy differed between the estrogen receptor-negative MDA-MB-231 and estrogen receptor-positive MCF-7 cells. The different hormonal characteristics of cancers may be closely associated with the synergistic effect of selenium on the actions of docetaxel, but we cannot offer any further explanation. Further studies are needed to explore and understand these differences.
In conclusion, we have identified that the combined therapy of selenium with docetaxel inhibits cell proliferation through apoptosis and cell arrest in the G2/M phase in MDA-MB-231 breast cancer cells. Chemotherapy combined with selenium may be beneficial for the treatment in some advanced breast cancer cases. Further studies are warranted to elucidate the molecular mechanism of the growth inhibition in combined treatment of breast cancer cells.
ACKNOWLEDGeMENTS
This work was supported by Konkuk University.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ko BS, Noh WC, Kang SS, Park BW, Kang EY, Paik NS, et al. Changing patterns in the clinical characteristics of korean breast cancer from 1996-2010 using an online nationwide breast cancer database. J Breast Cancer. 2012;15:393–400. doi: 10.4048/jbc.2012.15.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leclere B, Molinie F, Tretarre B, Stracci F, Daubisse-Marliac L, Colonna M, et al. Trends in incidence of breast cancer among women under 40 in seven European countries: a GRELL cooperative study. Cancer Epidemiol. 2013;37:544–549. doi: 10.1016/j.canep.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999;10:947–959. doi: 10.1091/mbc.10.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleeberg UR, Fink M, Tessen HW, Nennecke A, Hentschel S, Bartels S. Adjuvant therapy reduces the benefit of palliative treatment in disseminated breast cancer: own findings and review of the literature. Onkologie. 2013;36:348–356. doi: 10.1159/000351253. [DOI] [PubMed] [Google Scholar]
- 6.Valero V, Holmes FA, Walters RS, Theriault RL, Esparza L, Fraschini G, et al. Phase II trial of docetaxel: a new, highly effective antineoplastic agent in the management of patients with anthracycline-resistant metastatic breast cancer. J Clin Oncol. 1995;13:2886–2894. doi: 10.1200/JCO.1995.13.12.2886. [DOI] [PubMed] [Google Scholar]
- 7.Martin M, Segui MA, Anton A, Ruiz A, Ramos M, Adrover E, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010;363:2200–2210. doi: 10.1056/NEJMoa0910320. [DOI] [PubMed] [Google Scholar]
- 8.Baker J, Ajani J, Scotte F, Winther D, Martin M, Aapro MS, et al. Docetaxel-related side effects and their management. Eur J Oncol Nurs. 2009;13:49–59. doi: 10.1016/j.ejon.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton SJ. Review of selenium toxicity in the aquatic food chain. Sci Total Environ. 2004;326:1–31. doi: 10.1016/j.scitotenv.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Harris HR, Bergkvist L, Wolk A. Selenium intake and breast cancer mortality in a cohort of Swedish women. Breast Cancer Res Treat. 2012;134:1269–1277. doi: 10.1007/s10549-012-2139-9. [DOI] [PubMed] [Google Scholar]
- 11.Holmes MD, Stampfer MJ, Colditz GA, Rosner B, Hunter DJ, Willett WC. Dietary factors and the survival of women with breast carcinoma. Cancer. 1999;86:826–835. doi: 10.1002/(sici)1097-0142(19990901)86:5<826::aid-cncr19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Watrach AM, Milner JA, Watrach MA, Poirier KA. Inhibition of human breast cancer cells by selenium. Cancer Lett. 1984;25:41–47. doi: 10.1016/s0304-3835(84)80024-5. [DOI] [PubMed] [Google Scholar]
- 13.Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients. J Natl Cancer Inst. 2001;93:96–111. doi: 10.1093/jnci/93.2.96. [DOI] [PubMed] [Google Scholar]
- 14.Letavayova L, Vlckova V, Brozmanova J. Selenium: from cancer prevention to DNA damage. Toxicology. 2006;227:1–14. doi: 10.1016/j.tox.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Boucher F, Coudray C, Tirard V, Barandier C, Tresallet N, Favier A, et al. Oral selenium supplementation in rats reduces cardiac toxicity of adriamycin during ischemia and reperfusion. Nutrition. 1995;11(5 Suppl):708–711. [PubMed] [Google Scholar]
- 16.Fujieda M, Naruse K, Hamauzu T, Miyazaki E, Hayashi Y, Enomoto R, et al. Effect of selenium on Cisplatin-induced nephrotoxicity in rats. Nephron Exp Nephrol. 2006;104:e112–e122. doi: 10.1159/000094550. [DOI] [PubMed] [Google Scholar]
- 17.Francescato HD, Costa RS, Rodrigues Camargo SM, Zanetti MA, Lavrador MA, Bianchi MD. Effect of oral selenium administration on cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2001;43:77–82. doi: 10.1006/phrs.2000.0754. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawa K, Tsukada Y, Dohzono H, Koike K, Terashima Y. The effects of co-administration of selenium and cisplatin (CDDP) on CDDP-induced toxicity and antitumour activity. Br J Cancer. 1988;58:38–41. doi: 10.1038/bjc.1988.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan L, Jia X, Jiang X, Zhang Y, Tang H, Yao S, et al. In vitro study on the individual and synergistic cytotoxicity of adriamycin and selenium nanoparticles against Bel7402 cells with a quartz crystal microbalance. Biosens Bioelectron. 2009;24:2268–2272. doi: 10.1016/j.bios.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Baliga MS, Wang H, Zhuo P, Schwartz JL, Diamond AM. Selenium and GPx-1 overexpression protect mammalian cells against UV-induced DNA damage. Biol Trace Elem Res. 2007;115:227–242. doi: 10.1007/BF02685998. [DOI] [PubMed] [Google Scholar]
- 21.Kellen E, Zeegers M, Buntinx F. Selenium is inversely associated with bladder cancer risk: a report from the Belgian case-control study on bladder cancer. Int J Urol. 2006;13:1180–1184. doi: 10.1111/j.1442-2042.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 22.van den Brandt PA, Goldbohm RA, van't Veer P, Bode P, Dorant E, Hermus RJ, et al. A prospective cohort study on selenium status and the risk of lung cancer. Cancer Res. 1993;53:4860–4865. [PubMed] [Google Scholar]
- 23.Peters U, Chatterjee N, Church TR, Mayo C, Sturup S, Foster CB, et al. High serum selenium and reduced risk of advanced colorectal adenoma in a colorectal cancer early detection program. Cancer Epidemiol Biomarkers Prev. 2006;15:315–320. doi: 10.1158/1055-9965.EPI-05-0471. [DOI] [PubMed] [Google Scholar]
- 24.Weijl NI, Cleton FJ, Osanto S. Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev. 1997;23:209–240. doi: 10.1016/s0305-7372(97)90012-8. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann T, Leonhardt H, Kersting S, Albrecht S, Range U, Eckelt U. Reduction of postoperative lymphedema after oral tumor surgery with sodium selenite. Biol Trace Elem Res. 2005;106:193–203. doi: 10.1385/BTER:106:3:193. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M, Endo M, Shinohara F, Echigo S, Rikiishi H. Differential apoptotic response of human cancer cells to organoselenium compounds. Cancer Chemother Pharmacol. 2010;66:475–484. doi: 10.1007/s00280-009-1183-6. [DOI] [PubMed] [Google Scholar]
- 27.Luo H, Wang F, Bai Y, Chen T, Zheng W. Selenium nanoparticles inhibit the growth of HeLa and MDA-MB-231 cells through induction of S phase arrest. Colloids Surf B Biointerfaces. 2012;94:304–308. doi: 10.1016/j.colsurfb.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Rao L, Puschner B, Prolla TA. Gene expression profiling of low selenium status in the mouse intestine: transcriptional activation of genes linked to DNA damage, cell cycle control and oxidative stress. J Nutr. 2001;131:3175–3181. doi: 10.1093/jn/131.12.3175. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, Li F, Younes M, Liu H, Chen C, Yao Q. Reduced selenium-binding protein 1 in breast cancer correlates with poor survival and resistance to the anti-proliferative effects of selenium. PLoS One. 2013;8:e63702. doi: 10.1371/journal.pone.0063702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vekariya KK, Kaur J, Tikoo K. ERa signaling imparts chemotherapeutic selectivity to selenium nanoparticles in breast cancer. Nanomedicine. 2012;8:1125–1132. doi: 10.1016/j.nano.2011.12.003. [DOI] [PubMed] [Google Scholar]