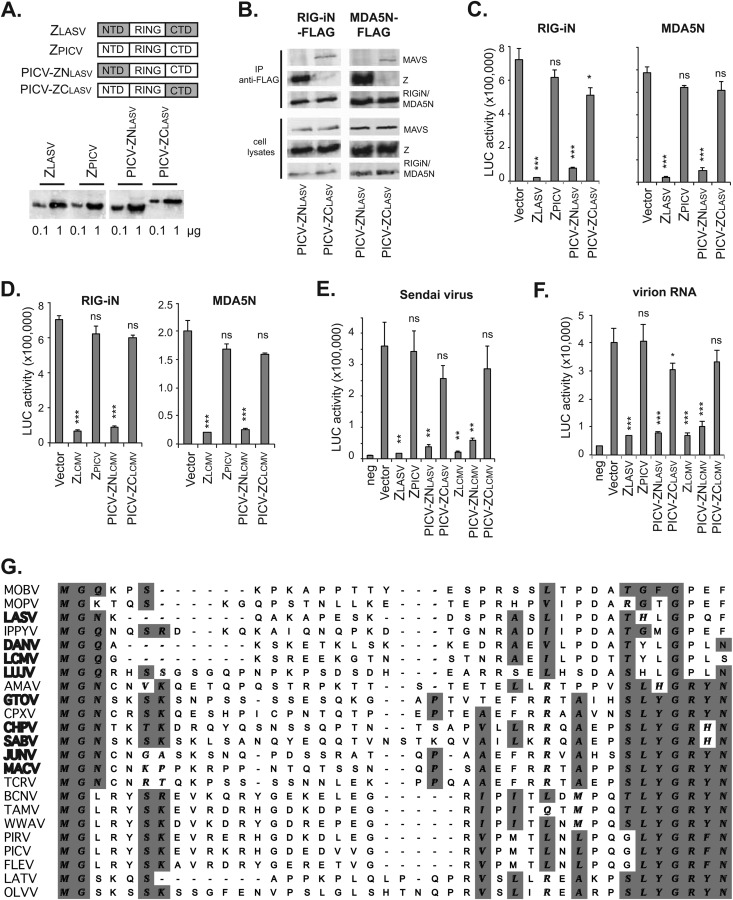
FIG 4.
The N-terminal domain (NTD) of pathogenic Z is a critical determinant for RLR binding and inhibition. (A) Diagrams showing the NTD, central RING domain, and CTD of LASV Z, PICV Z, and the chimeric Z proteins. Expression of these Z proteins in the transfected 293T cells was detected by Western blotting using anti-HA. (B) PICV-ZNLASV but not PICV-ZCLASV can interact with RIG-i and MDA5 and disrupt RLR-MAVS association in a coimmunoprecipitation assay as described in the Fig. 3 legend. (C) PICV-ZNLASV but not PICV-ZCLASV can inhibit RIG-iN- and MDA5N-induced IFN-β activation in a LUC-based promoter assay. (D) PICV-ZNLCMV but not PICV-ZCLCMV can inhibit RIG-iN- and MDA5N-induced IFN-β activation in a LUC-based promoter assay. (E) The Z proteins with a pathogenic NTD (ZLASV, ZLCMV, and chimeric PICV-ZNLASV and PICV-ZNLCMV) can inhibit Sendai virus-induced IFN-β production. (F) The Z proteins with a pathogenic NTD (ZLASV, ZLCMV, and chimeric PICV-ZNLASV and PICV-ZNLCMV) can inhibit virion RNA-induced IFN-β production. Each sample was compared to the vector control by statistical analysis using the Student t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not statistically significant. (G) Sequence comparison of arenavirus Z NTDs. The available Z protein sequences were obtained from GenBank. Alignment of multiple sequences was conducted using ClustalW in MacVector 12 software. Names of pathogenic viruses are shown in bold.