FIG 6.
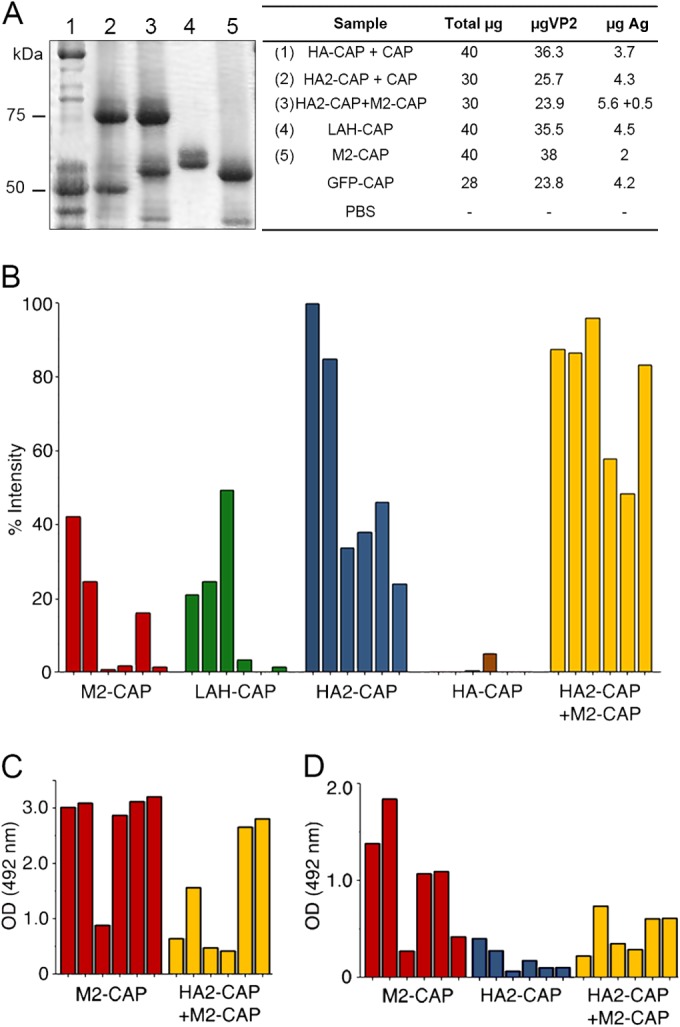
Antibody response after immunizations with influenza virus-derived chimeric assemblies. (A) SDS-PAGE of antigens (Ag) used for immunizations: Lanes: 1, HA-CAP–CAP; 2, HA2-CAP–CAP; 3, HA2-CAP–M2-CAP; 4, LAH-CAP; 5, M2-CAP. Molecular size markers (kDa) are indicated on the left. The table indicates the amounts of antigen per dose and mouse (μg). (B) Results of a dot blot assay of mouse antisera (1:300 dilution) after immunization with the indicated chimeric assembly to test for IgG antibody to inactivated influenza virus. Relative intensity was calculated as a percentage of the maximum signal recorded; each bar represents serum from a single mouse. (C) Results of an ELISA of M2-CAP and HA2-CAP–M2-CAP antisera (1:200 dilution) on plates coated with a synthetic M2 ectodomain peptide (SLLTEVETPIRNEWGCR). (D) Results for sera (1:200 dilution) from mice immunized with M2-CAP, HA2-CAP, or HA2-CAP–M2-CAP tested by ELISA on influenza virus-infected MDCK cells. Monoclonal anti-M2 antibody 14C2 (1 mg/ml) was diluted 1/2,000 as a control for panels B and C (optical density [OD] at 492 nm, 1.57 ± 0.26). GFP-CAP serum and PBS were used as negative controls (optical densities at 492 nm, 0.08 and 0.05, respectively).
