ABSTRACT
Epstein-Barr Virus (EBV) is a gammaherpesvirus that infects the majority of the human population and is linked to the development of multiple cancers, including nasopharyngeal carcinoma. Latent membrane protein 1 (LMP1) is considered the primary oncoprotein of EBV, and in epithelial cells it induces the expression and activation, or phosphorylation, of the epidermal growth factor receptor kinase. To identify effects on additional kinases, an unbiased screen of receptor tyrosine kinases potentially activated by LMP1 was performed. Using a protein array, it was determined that LMP1 selectively activates insulin-like growth factor 1 receptor (IGF1R). This activation takes place in fibroblast, epithelial, and nasopharyngeal cell lines that express LMP1 stably and transiently. Of note, LMP1 altered the phosphorylation, but not the expression, of IGF1R. The use of LMP1 mutants with defective signaling domains revealed that the C-terminal activating region 2 domain of LMP1 increased the mRNA expression and the secretion of the ligand IGF1, which promoted phosphorylation of IGF1R. IGF1R phosphorylation was dependent upon activation of canonical NF-κB signaling and was suppressed by IκBα and a dominant negative form of TRAF6. Inhibition of IGF1R activation with two small-molecule inhibitors, AG1024 and picropodophyllin (PPP), or with short hairpin RNA (shRNA) directed against IGF1R selectively reduced proliferation, focus formation, and Akt activation in LMP1-positive cells but did not impair LMP1-induced cell migration. Expression of constitutively active Akt rescued cell proliferation in the presence of IGF1R inhibitors. These findings suggest that LMP1-mediated activation of IGF1R contributes to the ability of LMP1 to transform epithelial cells.
IMPORTANCE EBV is linked to the development of multiple cancers in both lymphoid and epithelial cells, including nasopharyngeal carcinoma. Nasopharyngeal carcinoma is a major cancer that develops in specific populations, with nearly 80,000 new cases reported annually. LMP1 is consistently expressed in early lesions and continues to be detected within 50 to 80% of these cancers at later stages. It is therefore of paramount importance to understand the mechanisms through which LMP1 alters cell growth and contributes to tumorigenesis. This study is the first to determine that LMP1 activates the IGF1R tyrosine kinase by regulating expression of the ligand IGF1. Additionally, the data in this paper reveal that specific targeting of IGF1R selectively impacts LMP1-positive cells. These findings suggest that therapies directed against IGF1R may specifically impair the growth of EBV-infected cells.
INTRODUCTION
Epstein-Barr Virus (EBV) is a gammaherpesvirus transmitted through bodily fluids that infects both lymphocytes and oropharyngeal epithelial cells. It is estimated that greater than 90% of the human population are EBV carriers, and EBV infection is an etiological factor in the development of multiple cancers such as Burkitt lymphoma, Hodgkin lymphoma, gastric carcinoma, and nasopharyngeal carcinoma (NPC) (1). Roughly 78,000 new cases of NPC are reported each year, and there is a great need to develop improved treatments with increased specificity for malignant NPC cells (2).
Latent membrane protein 1 (LMP1) is considered the primary oncoprotein of EBV, and it consists of a short intracellular amino terminus, six transmembrane domains, and an intracellular carboxy-terminal tail containing 3 C-terminal activating regions (CTARs) that serve as docking sites for tumor necrosis factor receptor (TNF)-associated factors (TRAFs). The transmembrane domains of LMP1 promote protein aggregation and cytoskeletal remodeling, resulting in constitutive LMP1 activation and signaling. LMP1 is considered a viral mimetic of the tumor necrosis factor receptor (TNFR) CD40, and it activates multiple signaling pathways, including NF-κB, AKT, and mitogen-activated protein kinase (MAPK) signaling (1, 3) Specifically, CTAR1 binds TRAF1, -2, -3, and -5 and enhances AKT and MAPK signaling to promote rodent fibroblast transformation (4, 5). CTAR2 binds the TNF receptor-associated death domain protein (TRADD) and the TNF receptor-interacting protein (RIP) (1, 6).
Both CTAR1 and CTAR2 modulate cellular transcription via NF-κB signaling (7). Canonical NF-κB signaling, which is regulated by the inhibitor of NF-κB alpha (IκBα), is activated primarily by CTAR2, although CTAR1 may also promote canonical signaling (3, 7, 8). CTAR2 activates canonical NF-κB signaling through TRAF6, which binds CTAR2 indirectly via intermediates such as TRADD or RIP (9). In contrast, only CTAR1 can activate noncanonical NF-κB signaling through p100 and RelB, and LMP1 greatly increases the processing of p100 to p52 (8, 10–12). Many of the LMP1-associated TRAFs are ubiquitin ligases, which likely enables LMP1 effects on protein stability and localization (13).
Expression of LMP1 is particularly prevalent in NPC, where it is detected in 50 to 80% of tumors (14). LMP1 promotes epithelial cell survival and motility, and expression of LMP1 clinically correlates with NPC metastasis (1, 14). One contributing factor to cellular transformation is the ability of LMP1 to induce the expression and constitutive phosphorylation of the receptor tyrosine kinase (RTK) epidermal growth factor receptor (EGFR) (15). In epithelial cells, the CTAR1 domain of LMP1 signals through protein kinase C δ (PKCδ) and STAT3 to induce EGFR expression via a unique form of NF-κB signaling wherein p50 dimers bind to BCL3 to regulate gene transcription (10, 16–18). Although LMP1 increases the transcription of the EGFR ligand, transforming growth factor-α (TGF-α), TGF-α does not appear to be necessary for EGFR phosphorylation (19). Hence, it is unclear how LMP1 regulates EGFR activation.
EGFR is one of 58 human RTKs, which are divided into 20 families based on structural motifs and the similarities of their kinase domains (20). Broadly speaking, RTKs exist as transmembrane receptors which undergo dimerization and cross-phosphorylation upon ligand binding to enable downstream signaling. Murine models of NPC have determined that targeting EGFR decreases tumor growth and invasion (21, 22). Analysis of other cancer subtypes has revealed that tumors quickly develop resistance to EGFR monotherapy, but it may be possible to overcome this resistance using therapies that target multiple RTKs simultaneously (23–25). Collectively, these data suggest that targeting RTKs such as EGFR may provide novel mechanisms for treating NPC, either as individual therapeutics or in combination with EGFR inhibitors. Recent studies have suggested that LMP1 may regulate the expression of RTKs such as RON, but an unbiased screen of RTKs activated by LMP1 has yet to be performed (26, 27). The activation of 49 different RTKs was therefore analyzed in the presence and absence of LMP1. The resulting data indicate a novel mechanism by which LMP1 may transform cells and offer the opportunity to develop new therapies for the selective treatment of LMP1-positive NPC.
MATERIALS AND METHODS
Cell lines, C15 tumors, plasmid constructs, and small-molecule inhibitors.
Rat1 and 293T cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Gibco, NY, USA) and 1% penicillin-streptomycin. MCF10a cells were cultured in DMEM-F12 (Gibco) with 5% horse serum, 1% penicillin-streptomycin, 0.5 μg/ml hydrocortisone, 100 ng/ml cholera toxin, 10 μg/ml insulin, and 20 ng/ml EGF. NP69 cells were grown in keratinocyte serum-free medium supplemented with 5% FBS, 1% penicillin-streptomycin, 25 μg/ml bovine pituitary extract, and 0.2 ng/ml EGF. C666 cells were grown on fibronectin-coated plates with RPMI (Gibco) supplemented with 10% FBS and 1% penicillin-streptomycin. C15 tumors were passaged serially in nude mice. Tumors were dissected and incubated with NP-40 lysis buffer (1% NP-40, 150 nM NaCl, 50 mM Tris [pH 7.4], 5 mM EDTA, and 10% glycerol) for 1 h. Remaining tissue debris was spun down and extracted, while the supernatant was kept for immunoblotting analysis. Cells were also lysed in NP-40 lysis buffer. Protease and phosphatase inhibitors (Sigma-Aldrich, MO, USA) were added to NP-40 lysis buffer before each use.
LMP1 constructs containing hemagglutinin (HA)-tagged wild-type (WT) LMP1, LMP1 A5, or LMP1 378 in the pBabe-puro backbone have been described previously (5). A pLKO.1 plasmid containing scrambled short hairpin RNA (shRNA) (plasmid 1864) and a pLNCX plasmid containing myristoylated Akt (myr-Akt) (plasmid 9005) were obtained from Addgene (MA, USA) (28, 29). pLKO.1 plasmids with shRNA directed against IGF1R (shIGF-1, TRCN0000121297; shIGF-2, TRCN0000121301) were obtained from the Lenti-shRNA Core Facility at the University of North Carolina, Chapel Hill (NC, USA). The superrepressor (SR) IκBα construct in the pcDNA3-zeo backbone was made as described previously (30). A dominant negative TRAF6 (dnTRAF6) construct and a Myc-tagged dominant negative TRAF2 construct, both in the pBabe-puro backbone, were made as described previously (5, 31, 32). Prior to transfection, cells were seeded to 50% confluence in a 10-cm tissue culture dish. Cells were transfected with 6 μg of DNA and 10 μl Lipofectamine 2000 (Invitrogen, NY, USA) for 5 h.
The small-molecule inhibitor picropodophyllin (PPP) was purchased from Santa Cruz Biotechnology (TX, USA), and AG1024 was obtained from SelleckChem (TX, USA). Both inhibitors were reconstituted in dimethyl sulfoxide (DMSO). Where indicated, cells were treated with DMSO as a vehicle control. The DMSO vehicle control was always used at a concentration equal to the highest concentration of experimental inhibitors (usually equivalent to 20 μM AG1024).
Immunoprecipitation, immunoblotting, antibodies, and RTK arrays.
Immunoprecipitations to detect phospho-IGF1R expression were conducted using 1 mg of protein harvested 2 days after transfection. Lysates were either immunoprecipitated with anti-IGF1R (Santa Cruz Biotechnology) and probed with antiphosphotyrosine (anti-pTyr) (Cell Signaling Technology, MA, USA) or immunoprecipitated with anti-pTyr and probed with anti-IGF1R (Cell Signaling Technology). Where indicated, IgG (Jackson ImmunoResearch, PA, USA) was used to immunoprecipitate lysates as a negative control. Other antibodies used for immunoblotting included anti-LMP1 CS1-4 (Dako, CA, USA); anti-HA (Covance, NC, USA); anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase), anti-IκBα, anti-Myc, and anti-TRAF6 (Santa Cruz Biotechnology); and anti-phospho-Akt and anti-Akt (Cell Signaling Technology). Immunoblotting was conducted as described previously (33). Briefly, protein samples (either immunoprecipitations or 25 μg of total cell lysates as determined by the Bradford assay) in Laemmli loading buffer were loaded onto a 12% agarose SDS-polyacrylamide gel. Gels were transferred onto polyvinylidene difluoride (PVDF) membranes (Fisher Scientific, PA, USA) prior to antibody analysis.
A neutralizing anti-IGF1R was obtained from R&D Systems (MN, USA). The neutralizing anti-IGF1R was used in cell medium diluted 1:2 with Opti-MEM (Gibco). Where indicated, cells were treated with control goat IgG (Jackson ImmunoResearch) instead of neutralizing anti-IGF1R. The control IgG was used at concentrations equal to the highest dose of anti-IGF1R (usually 8 μg/ml).
The relative intensities of bands in immunoblots were assessed with ImageJ software (34). To determine LMP1 expression in cell lines relative to C15 tumors, LMP1 expression was normalized to GAPDH bands for a given sample. The normalized LMP1 expression in cell lines was then divided by the average normalized LMP1 expression in C15 xenografts. To quantitate Akt phosphorylation, the amount of phospho-Akt was normalized to GAPDH bands. Relative Akt phosphorylation was determined by dividing the normalized Akt phosphorylation for every experimental treatment by the normalized Akt phosphorylation for untreated cells. The fraction of relative Akt phosphorylation for LMP1-positive cells was then divided by the fraction of relative Akt phosphorylation for pBabe vector control cells. RTK phosphoarrays were purchased from R&D Systems and were used with 500 μg of protein in accordance with the manufacturer's instructions. A detailed list of RTKs examined in the array may be found at http://www.rndsystems.com/Products/ARY001B.
Proliferation and focus formation assays.
Cell proliferation was assessed using a variant of the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay called the CellTiter 96 aqueous one-solution cell proliferation assay (Promega, WI, USA) in accordance with the manufacturer's instructions. Briefly, 1,000 cells were seeded in 96-well plates. Cells treated with shRNA constructs were seeded 1 day after transfection. After 4 h, a T0 time point was read to assess cell seeding, and the indicated treatments (small-molecule inhibitors or neutralizing anti-IGF1R) were added. The cell number was assessed again 48 h later, and the ratio of the number at T48 to that at T0 was obtained. For pBabe- or LMP1-positive cells, the proliferation of treated cells was taken as a fraction of the proliferation of untreated cells. The proliferating fraction of LMP1-positive cells was then divided by the proliferating fraction of pBabe vector control cells.
Focus formation was assessed by seeding 500 cells in 6-well dishes and allowing them to grow for 10 days before staining with Coomassie dye. As with proliferation assays, cells transfected with shRNA were seeded 1 day after transfection. Cells were treated with small-molecule inhibitors starting 4 h after seeding and throughout the remainder of the experiment. Focus number and size were quantitated with ImageJ software (34). Where indicated, the focus number or size of treated cells was taken as a fraction of the focus size or number of untreated cells. The fraction of LMP1-positive foci was then taken relative to the fraction of pBabe foci.
Migration assays.
Cell migration was measured using 8-μm 24-well transwell assays from BD Bioscience (CA, USA). Briefly, 20,000 Rat1 cells in 0.1% FBS were seeded in the top chamber and allowed to migrate toward 10% FBS in the bottom chamber for 6 h. Where indicated, the small-molecule inhibitors PPP and AG1024 were placed in both the top and bottom chambers at the time of cell seeding. Cells that did not migrate through the transwell were scraped off, while migrating cells were stained. Four random fields were counted for each transwell to determine the number of migrating cells. Where indicated, the number of treated migrating cells was assessed as a fraction of the number of untreated migrating cells. The fraction of migrating LMP1-positive cells was then taken relative to the fraction of migratory pBabe vector control cells.
qRT-PCR.
RNA was harvested from cells using the RNeasy Plus minikit from Qiagen (CA, USA). Quantitative real-time PCR (qRT-PCR) was performed using 100 ng of RNA and the QuantiFast SYBR green RT-PCR kit from Qiagen according to the manufacturer's instructions. Primers were designed using Primer-BLAST and were ordered from Eurofins Genomics (AL, USA). Primers used were as follows: IGF1R-F, GAG TGG AGA AAT CTG CGG GC; IGF1R-R, TCG ATC AGG GTG CAG TTC TC; GAPDH-F, TGC ACC ACC AAC TGC TTA GC; GAPDH-R, GAG GGG CCA TCC ACA GTC TT; IGF1-F, AAT CAG CAG TCT TCC AAC CCA; IGF1-R, CAC AGC GCC AGG TAG AAG AG; IGF2-F, CCC GTC GCA CAT TCG GC; IGF2-R, GGG ATT CCC ATT GGT GTC TGG A; LMP1-F, CAT AGC CCT AGC GAC TCT GC; and LMP1-R, AAA GGG CTC CAA GTG GAC AG.
ELISA.
The IGF1 Quantikine enzyme-linked immunosorbent assay (ELISA) kit was obtained from R&D Systems and used in accordance with the manufacturer's instructions. Briefly, 50,000 cells were seeded in 6-well dishes and allowed to grow for 3 days. Cell medium was harvested, and 2 μl of medium was used in the ELISA. Readings from “control” medium without cells were subtracted from experimental medium readouts. Where indicated, the relative IGF1 concentration of LMP1-positive cells was divided by the IGF1 concentration of pBabe control cells.
RESULTS
LMP1 expression increases IGF1R activation.
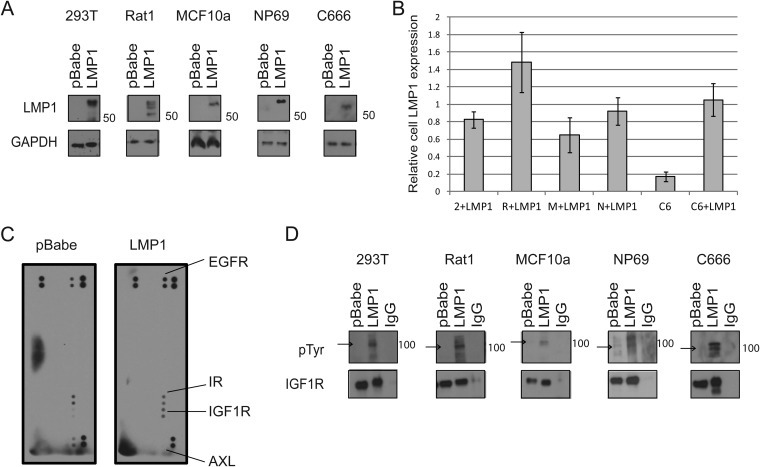
Constructs containing LMP1 or pBabe empty vector control were expressed in multiple cell lines (Fig. 1A). LMP1 was transiently transfected into 293T embryonic kidney cells, C666 nasopharyngeal carcinoma cells, and NP69 premalignant nasopharyngeal epithelial cells. LMP1 was stably expressed in immortalized, nontransformed MCF10a epithelial cells and Rat1 rodent fibroblasts (Fig. 1A). To confirm that the transfected cell lines expressed LMP1 at physiologically relevant levels, the expression of LMP1 in transfected cells was compared to LMP1 expression in C15 xenograft tumors. C15 tumors are serially passaged in vivo models of NPC that contain the EBV genome and consistently express LMP1 (35) (Fig. 1B). Immunoblots were used to analyze LMP1 expression in 3 samples of each cell line with and without LMP1 transfection and in lysates from C15 tumors. LMP1 expression was normalized to GAPDH for each sample, and the ratio of LMP1 expression was then taken relative to the average LMP1 expression in C15 tumors for each cell line (Fig. 1B). LMP1 expression was not detected in parental 293T, Rat1, NP69, or MCF10a cells (data not shown). In contrast, the C666 cell line contains the EBV genome and is thought to express LMP1 at low levels (33, 36). Parental C666 cells expressed approximately 20% as much LMP1 as C15 tumors (Fig. 1B). As expected, transfection increased LMP1 expression in all cell lines. Importantly, transfected cell lines expressed an amount of LMP1 similar to that for C15 tumors (average range, 60 to 150% of LMP1 found in C15 tumors), indicating that the cell lines were expressing LMP1 at physiologically relevant levels (Fig. 1B).
FIG 1.
LMP1 increases IGF1R activation. (A) Immunoblots for LMP1 expression in transiently transfected cell lines (293T, C666, and NP69) and in stably transfected cell lines (MCF10a and Rat1). (B) Immunoblots were run to examine LMP1 expression in the following cell lines with (+LMP1) or without LMP1 transfection: 293T (2) + LMP1, Rat1 (R) + LMP1, MCF10a (M) + LMP1, NP69 (N) + LMP1, C666 (C), and C666 (C) + LMP1. Three samples were examined, and expression of LMP1 was calculated relative to LMP1 expression in C15 xenograft tumors. Average LMP1 expression is depicted. (C) 293T lysates expressing LMP1 or pBabe empty vector were added to arrays spotted in duplicate with antibodies against 49 human RTKs. Arrays were probed with anti-pTyr to detect specific RTK phosphorylation; darker spots indicate stronger activation. Spots in the upper left, upper right, and lower right are positive controls, while that in the lower left is a negative control. (D) The indicated cells were lysed and immunoprecipitated with anti-IGF1R or IgG. The resulting immunoprecipitates were probed with anti-pTyr or with anti-IGF1R. Beta chains of IGF1R are 97 kDa (arrows), and alpha chains of IGF1R are 130 kDa.
Two days after transfection, lysates from 293T cells expressing LMP1 or pBabe were incubated with protein arrays that were spotted in duplicate with antibodies against 49 distinct RTKs. Arrays were probed with antiphosphotyrosine (anti-pTyr) to determine which RTKs were specifically activated in the presence of LMP1 (Fig. 1C). On the array, darker spots were indicative of higher levels of phosphorylation, and positive controls were spotted in the upper left, upper right, and lower right corners, while the lower left corner served as a negative control. RTKs that were clearly phosphorylated were identified and labeled (Fig. 1C).
In keeping with previous reports, expression of LMP1 increased the phosphorylation of EGFR (15–17). At the same time, LMP1 decreased the activation of AXL and increased the phosphorylation of insulin-like growth factor 1 receptor (IGF1R). Interestingly, activation of insulin receptor (IR), which is closely related to IGF1R, was not altered by LMP1 (Fig. 1C). IGF1R is a tetrameric RTK implicated in the development of multiple cancer subtypes, and its signaling capabilities and role in cellular transformation have been extensively reviewed elsewhere (37, 38). Mature IGF1R contains two extracellular alpha subunits linked by disulfide bonds and two membrane-spanning beta subunits that contain intracellular kinase domains and docking sites for adaptor proteins. The alpha subunits of IGF1R have a molecular mass of roughly 130 kDa, while the beta subunits have a molecular mass of approximately 97 kDa and frequently generate the predominant bands detected via immunoblotting (38). Most antibodies that detect phospho-IGF1R (pIGF1R) also cross-react with pIR. To recapitulate the detection method in the protein arrays, cell lysates were immunoprecipitated with anti-IGF1R and probed with anti-pTyr to specifically detect pIGF1R. This approach demonstrated that LMP1 increased the activation of IGF1R in 293T, C666, NP69, MCF10a, and Rat1 cells (Fig. 1D). The C666 cell line contains the EBV genome and is thought to express LMP2 and EBV RNAs, with trace levels of LMP1 (33, 36). Expression of exogenous LMP1 in C666 cells induced IGF1R activation in the presence of the EBV genome, indicating that LMP1 can regulate pIGF1R levels in the context of EBV infection. Elevated pIGF1R was detected in cell lines stably (MCF10a and Rat1) and transiently (293T, C666, and NP69) expressing LMP1, indicating that the effects of LMP1 on IGF1R are consistent (Fig. 1D).
Inhibiting IGF1R reduces cell proliferation, focus formation, and Akt phosphorylation but does not influence cell migration.
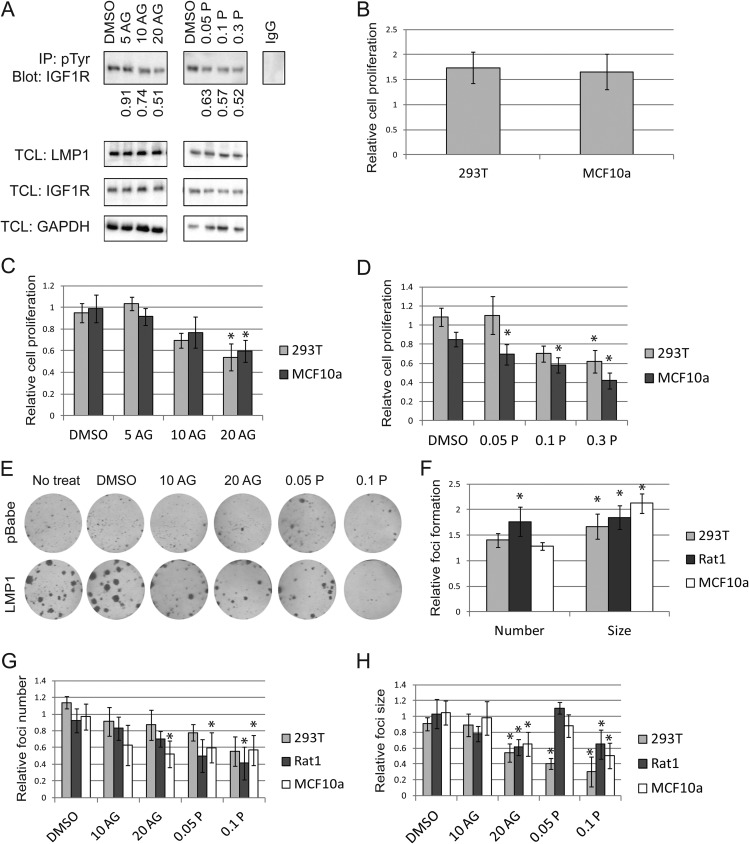
The small-molecule inhibitors AG1024 and picropodophyllin (PPP) are designed to inhibit IGF1R phosphorylation, with 50% inhibitory concentration (IC50) values of 7 μM and 40 to 70 nM, respectively (39–42). Cells treated with AG1024 or PPP were immunoprecipitated with anti-pTyr and immunoblotted with anti-IGF1R. This approach provided a more sensitive mechanism to detect pIGF1R while reinforcing the results obtained from reciprocal immunoprecipitations used for Fig. 1D. As expected, AG1024 and PPP reduced LMP1-induced IGF1R activation in a dose-dependent manner but did not alter IGF1R or LMP1 expression (Fig. 2A). PPP inhibited IGF1R phosphorylation with an IC50 roughly equal to 50 nM, which is in agreement with prior results (39). Although AG1024 reduced IGF1R phosphorylation, its IC50 was approximately 20 μM (Fig. 2A). Previous studies have, however, determined that AG1024 targets IR with an IC50 of roughly 57 μM (40). Since IR is the RTK most closely related to IGF1R, it is possible that AG1024 is still selectively targeting IGF1R at doses of 20 μM (40). Both PPP and AG1024 were subsequently used in parallel to ensure that experimental observations were not skewed by off-target effects of the small-molecule inhibitors.
FIG 2.
LMP1 promotes cell proliferation and focus formation via IGF1R. (A) LMP1-expressing 293T cells were incubated with the indicated micromolar concentrations of AG1024 (AG) or PPP (P) for 1 day. Cell lysates were immunoprecipitated (IP) with anti-pTyr and probed with anti-IGF1R to specifically detect phospho-IGF1R. Numerical values indicate fold reduction in pIGF1R compared to that in a dimethyl sulfoxide (DMSO) vehicle control. In parallel, total cell lysates (TCLs) were probed for IGF1R, LMP1, and GAPDH. (B to D) MTS assays were used to assess the proliferation of LMP1-positive cells relative to pBabe cell proliferation over a 2-day period (B). In the same time span, cells were exposed to DMSO vehicle control, AG1024 (C), or PPP (D), and proliferation was measured. (E to H) MCF10a cells were sparsely seeded and given the indicated treatment for 10 days before foci were stained and quantitated (E). The focus number and size of LMP1-expressing cells were calculated relative to those for pBabe vector controls (F). Four hours after seeding, cells were treated with AG1024 or PPP, and the relative number (G) and size (H) of foci were subsequently assessed. In panels C, D, G, and H, the fraction of treated cell growth or focus formation was calculated relative to that for untreated cells. The proportion of LMP1-positive cells was then calculated relative to that for pBabe vector control cells. All drug doses are micromolar. *, LMP1-positive cells are significantly different than pBabe cells (P < 0.05).
Compared to pBabe vector control cells, expression of LMP1 increased the proliferation of 293T and MCF10a cells by approximately 50% as measured by MTS assay (Fig. 2B). To take this fundamental proliferation difference into account, the proliferation of cells treated with AG1024 or PPP was normalized to the proliferation of untreated cells. These calculations enabled direct comparison of the amount of proliferating LMP1-positive cells and the amount of proliferating pBabe vector control cells. The fraction of LMP1-positive proliferating cells was then calculated relative to the fraction of proliferating pBabe control cells. Treatment with AG1024 or PPP selectively reduced the proliferation of LMP1-positive cells, although PPP decreased cell proliferation more effectively than AG1024 (Fig. 2C and D). Recent studies as well as unpublished data from our lab indicate that expression of LMP1 can alter the metabolism of cells, which may affect the results of traditional proliferation assays that measure levels of metabolic by-products (43). Focus formation assays were therefore used to simultaneously assess loss of contact inhibition and cell growth independently of metabolic by-products. Briefly, a small number of cells were seeded and allowed to grow for 10 days. Both the size and number of resulting colonies are indicative of cell proliferation, while the size of the colonies also correlates with loss of contact inhibition. In this way, two hallmarks of cancer development may be assessed concurrently. As expected, LMP1 dramatically increased the size and number of foci formed in 293T, MCF10a, and Rat1 cells (Fig. 2E and F). Inhibition of IGF1R selectively decreased the size and number of foci formed by LMP1-positive cells relative to those formed by vector control cells (Fig. 2G and H). Both focus size and focus number were reduced by IGF1R inhibitors in a dose-dependent manner, indicating that pIGF1R selectively promoted focus development in all LMP1-positive cells (Fig. 2G and H).
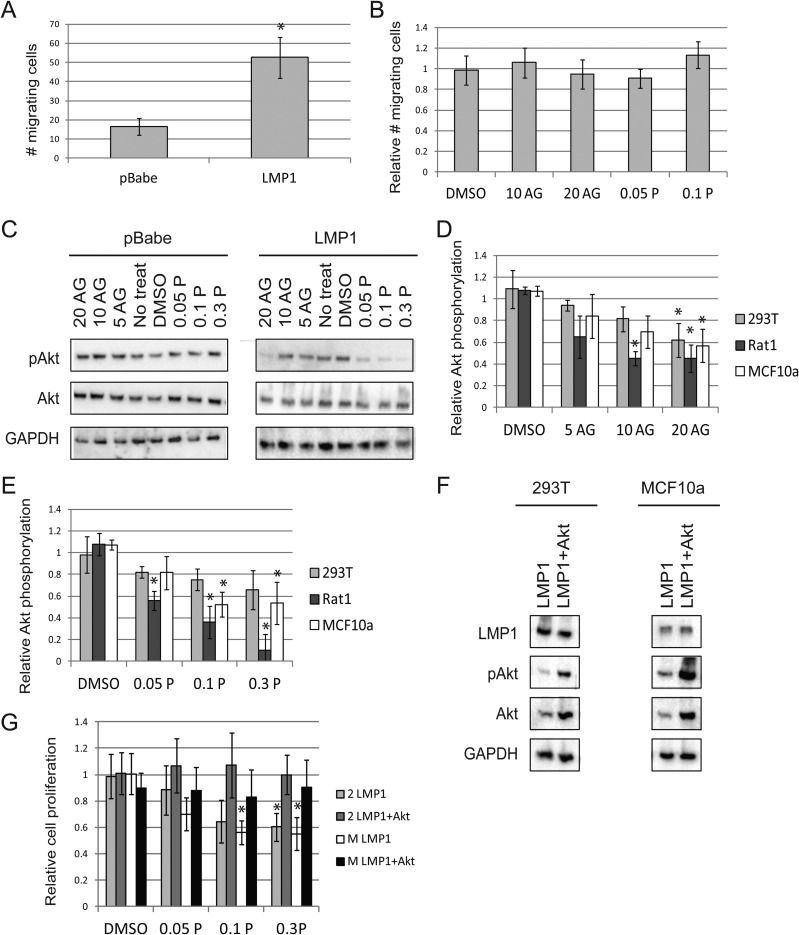
LMP1 is capable of increasing cell motility and promoting metastasis (5, 44). In agreement with these observations, LMP1 expression increased Rat1 cell migration by roughly 3-fold compared to pBabe vector control cells in a transwell assay (Fig. 3A). Treatment with AG1024 or PPP did not inhibit the migration of LMP1-expressing cells relative to that of vector control cells (Fig. 3B). Thus, IGF1R signaling does not appear to regulate LMP1-induced migration in rodent fibroblasts.
FIG 3.
Inhibiting IGF1R reduces Akt phosphorylation but does not alter LMP1-mediated migration. (A) The number of cells that migrated through a transwell system over a 6-h span was assessed for Rat1 cells expressing LMP1 or pBabe vector control. (B) Rat1 cells were treated with DMSO vehicle control, AG1024, or PPP and allowed to migrate through a transwell system. The migration of LMP1-positive cells is shown relative to that of pBabe control cells. (C to E) In MCF10a cells, immunoblots were used to assess the activation of Akt after AG1024 or PPP treatment for 1 day (C). The activation of Akt relative to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was calculated for AG1024 (D) and PPP (E). The fraction of Akt activation in treated cells was taken relative to that in untreated cells. Ratios of Akt activation in LMP1-positive cells relative to that in LMP1-negative cells were then calculated. (F) Immunoblots for expression of myr-Akt (+Akt) in LMP1-expressing 293T and MCF10a cells. (G) The MTS assay was used to assess cell proliferation of 293T (2) and MCF10a (M) cells in the presence of the indicated micromolar doses of PPP. Proliferation of LMP1 cells with or without myr-Akt is depicted relative to that of vector control cells. *, LMP1-positive cells were significantly different than vector control cells (P < 0.05).
Upon activation, the intracellular domains of IGF1R become cross-phosphorylated and serve as docking sites for adaptor proteins such as insulin receptor substrates (IRSs) or Shc. Although IGF1R can activate multiple downstream signaling pathways, it is traditionally associated with Akt signaling, and it has been shown that phosphatidylinositol 3-kinase (PI3K) can interact with either IRSs or IGF1R itself to induce Akt phosphorylation (37). In the presence of serum, expression of LMP1 did not significantly affect pAkt levels; however, treatment with AG1024 or PPP selectively reduced Akt phosphorylation in LMP1-positive cells relative to that in LMP1-negative cells (Fig. 3C to E and data not shown). These data indicate that LMP1 expression promotes Akt phosphorylation through IGF1R activation, while pBabe control cells activate Akt phosphorylation via a different mechanism.
To determine whether IGF1R regulated cell proliferation via pAkt, a myristoylated Akt (myr-Akt) construct was transfected into LMP1-expressing 293T and MCF10a cells (Fig. 3F). The myristoylation targets Akt to the plasma membrane, allowing Akt to become constitutively active (29). The presence of myr-Akt prevented PPP from selectively decreasing the proliferation of LMP1-expressing cells, suggesting that the loss of Akt phosphorylation generated by targeting IGF1R regulates the observed changes in cell proliferation (Fig. 3G).
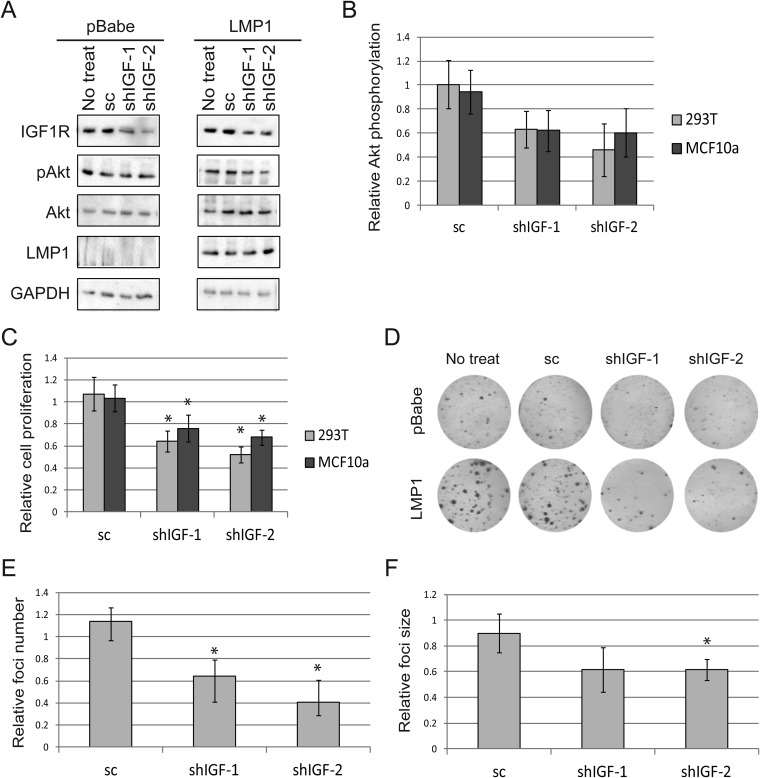
Effects of small-molecule IGF1R inhibitors are recapitulated by shRNA.
As mentioned above, AG1024 and PPP are small-molecule inhibitors designed to prevent IGF1R activation. Although AG1024 and PPP are thought to specifically inhibit IGF1R, it is possible that these small-molecule inhibitors may have other, off-target effects. To verify that LMP1 specifically regulates cell proliferation and Akt activation via IGF1R, two different shRNAs (shIGF-1/2) were used to knock down expression of IGF1R. These shRNAs reduced IGF1R expression compared to that in scrambled shRNA control cells or untreated cells and did not affect LMP1 expression (Fig. 4A). IGF1R knockdown was effective in both LMP1-expressing and pBabe vector control cells, yet targeting IGF1R selectively reduced Akt phosphorylation in LMP1-positive cells compared to LMP1-negative cells (Fig. 4A and B). Due to measurement variability, the decrease in Akt activation in LMP1-expressing cells was not significant compared to activation in vector control cells (Fig. 4B). Proliferation of LMP1-positive cells was selectively and significantly decreased by IGF1R knockdown relative to that of vector control cells (Fig. 4C). Importantly, the degree to which IGF1R knockdown impacted Akt phosphorylation paralleled the decrease in cell proliferation, suggesting that IGF1R regulates cell proliferation through Akt activation (Fig. 4B and C). In agreement with previous results, LMP1 expression increased the size and number of foci formed relative to those in pBabe control cells, and these effects were specifically abrogated by loss of IGF1R expression (Fig. 4D to F).
FIG 4.
The use of shRNA verifies that targeting IGF1R specifically reduces Akt activation, cell proliferation, and focus formation in LMP1-positive cells. (A and B) Two different shRNAs (shIGF-1 and shIGF-2) were used to knock down IGF1R in MCF10a cells expressing LMP1 or pBabe. Scrambled shRNA (sc) was used as a control. Activation of Akt was subsequently assessed (A). The fraction of Akt activation relative to a GAPDH loading control in LMP1-expressing cells was determined relative to LMP1-negative cells (B). (C) MTS assays measured cell proliferation of LMP1-positive cells compared to pBabe cells after the indicated treatment. (D to F) After transfection with shRNA or sc, 293T cells were sparsely seeded and allowed to form foci for 10 days (D). The relative number (E) and size (F) of foci were then calculated for LMP1-expressing cells relative to pBabe cells. In panels B, C, E, and F the fraction of treated cell growth or focus formation was calculated relative to that for untreated cells. The proportion of LMP1-positive cells was then calculated relative to that for pBabe vector control cells. *, LMP1-positive cells were significantly different than pBabe control cells (P < 0.05).
LMP1 mediates IGF1R activation via ligand IGF1.
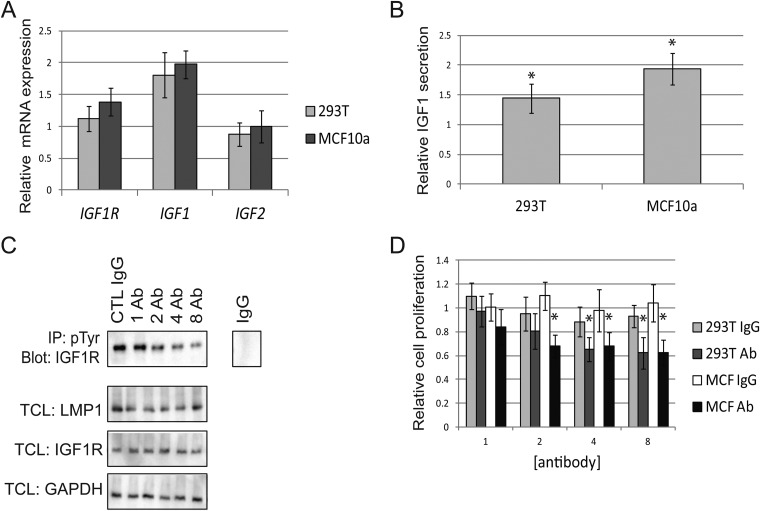
Activating IGF1R mutations in cancer have not yet been identified. Instead, IGF1R may be activated by overexpression or by ligands such as IGF1 or IGF2. In many cancer subtypes, cells promote IGF1R activation by secreting ligands in an autocrine manner (37). SYBR green qRT-PCR was used to compare the expression of mRNA in LMP1-positive cells to mRNA expression in pBabe control cells. These data revealed that LMP1 expression did not alter the expression of the IGF1R or IGF2 gene relative to that in pBabe vector control cells (Fig. 5A). In contrast, mRNA of IGF1 was elevated by roughly 2-fold in LMP1-positive cells compared to LMP1-negative cells (Fig. 5A). ELISAs verified that increased mRNA levels of IGF1 correlated with elevated secretion of IGF1 in LMP1-positive cells relative to vector control cells (Fig. 5B). The agreement between the ELISAs and the qRT-PCR data suggests that LMP1 regulates IGF1 secretion via de novo synthesis of the growth factor (Fig. 5A and B). To verify that LMP1 regulated IGF1R activation via ligand secretion, a neutralizing anti-IGF1R was used to prevent ligand binding. As a control, cells were also treated with goat IgG to verify the specificity of the effects induced by anti-IGF1R. Incubation with increasing concentrations of anti-IGF1R over a 24-hour period reduced IGF1R tyrosine phosphorylation in LMP1-positive cells, while incubation with IgG controls did not alter pIGF1R levels (Fig. 5C). Neither the neutralizing antibody nor the IgG control altered expression of IGF1R or LMP1 (Fig. 5C). Use of IgG controls did not alter the proliferation of LMP1-positive 293T or MCF10a cells relative to that of pBabe vector control cells (Fig. 5D). Neutralizing anti-IGF1R, however, selectively decreased the proliferation of LMP1-expressing cells relative to that of LMP1-negative cells (Fig. 5D).
FIG 5.
LMP1 regulates IGF1R activation via ligand IGF1. (A) qRT-PCR was used to assess the expression of IGF1R, IGF1, and IGF2. (B) ELISAs measured the secretion of IGF1. In panels A and B, the ratio of LMP1-positive cells relative to LMP1-negative cells is depicted. (C) LMP1-expressing 293T cell lysates were immunoprecipitated with anti-pTyr and probed with anti-IGF1R to detect pIGF1R after treatment with control goat IgG (CTL IgG) or with neutralizing anti-IGF1R (Ab) for 24 h. In parallel, total cell lysates (TCLs) were probed for IGF1R, LMP1, and GAPDH. (D) Proliferation of 293T or MCF10a (MCF) cells when treated with neutralizing anti-IGF1R (Ab) or IgG control for 48 h. The fraction of treated cells relative to cells without treatment was calculated, and proliferation of LMP1-positive cells was taken relative to that of LMP1-negative cells. All antibody concentrations are μg/ml. *, the value for LMP1-positive cells is significantly different than that for pBabe control cells (P < 0.05).
The CTAR2 domain of LMP1 regulates IGF1R activation.
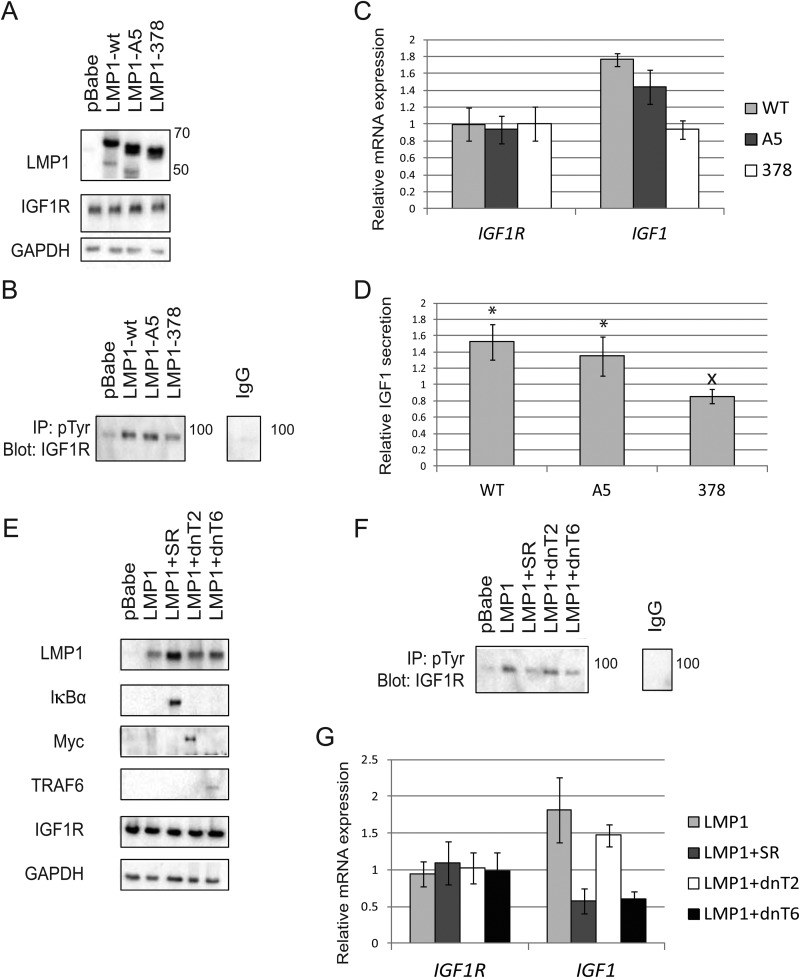
LMP1 contains 2 CTAR domains (CTAR1 and -2) that regulate the majority of LMP1-mediated signaling. LMP1 mutants without a functional CTAR1 or CTAR2 domain have been previously described (5). Briefly, the functionality of CTAR1 was disrupted by mutating the TRAF-binding PQQAT region to AAAAA to generate the A5 LMP1 mutant. CTAR2 was deleted from LMP1 by truncating the protein at amino acid 378 to generate the 378 LMP1 mutant (5). Both the A5 and the 378 LMP1 mutants are HA tagged, and the deletion within the 378 mutant makes it slightly smaller than the predicted 66-kDa LMP1 (Fig. 6A). The wild-type (WT) LMP1 construct was tagged with both FLAG and HA, which increased the molecular mass of WT LMP1 to slightly above the predicted 66-kDa mass (Fig. 6A). Transfection of the LMP1 constructs or pBabe control vectors into cells did not alter the expression of IGF1R at the protein level (Fig. 6A). In contrast, LMP1 378 selectively reduced pIGF1R levels compared to WT LMP1 or LMP1 A5 (Fig. 6B). Additionally, SYBR green qRT-PCR demonstrated that LMP1 378 decreased the mRNA levels of IGF1R ligand IGF1 (Fig. 6C). In keeping with previous findings, ELISAs confirmed that WT LMP1 and LMP1 A5 significantly increased IGF1 secretion relative to that for pBabe controls, and LMP1 378 significantly reduced the secretion of IGF1 relative to that for WT LMP1 (Fig. 6D). Interestingly, LMP1 A5 also slightly reduced the mRNA expression and secretion of IGF1 compared to that for WT LMP1, but LMP1 A5 did not impact IGF1 to the same extent as LMP1 378 (Fig. 6C and D).
FIG 6.
The CTAR2 domain of LMP1 controls IGF1 secretion and IGF1R activation via NF-κB signaling. (A) Wild-type (WT) LMP1 as well as the A5 and 378 mutants of LMP1 were expressed in 293T cells. WT LMP1 has FLAG and HA tags, while LMP1 378 is missing a fragment of the C terminus; these alterations make them slightly larger and smaller than the predicted 66-kDa LMP1, respectively. The protein expression of IGF1R was assessed in parallel. (B) 293T cells were transfected with WT LMP1 or the indicated mutant. Lysates were immunoprecipitated with anti-pTyr and immunoblotted with anti-IGF1R to specifically detect IGF1R phosphorylation. (C) qRT-PCR analyzing transcription of IGF1R and IGF1 with the indicated LMP1 constructs compared to pBabe controls. (D) ELISAs measured secretion of IGF1 with the indicated LMP1 constructs compared to pBabe controls. (E) Immunoblots depicting 293T cells transfected with HA-tagged LMP1 (66 kDa), Myc-tagged dominant negative TRAF2 (dnT2) (57 kDa), dominant negative TRAF6 (dnT6) (60 kDa), or HA-tagged IκBα superrepressor (SR) (35 kDa). (F) Two days after transfection, the indicated cell lysates were immunoprecipitated with anti-pTyr and probed with anti-IGF1R to specifically detect phospho-IGF1R. (G) qRT-PCR describing transcription of IGF1R and IGF1 with the indicated constructs. All results compare gene expression of LMP1-positive cells to that of pBabe control cells. *, LMP1-expressing cells were significantly different from pBabe control cells (P < 0.05). x, the indicated value is significantly different from that for WT LMP1 (P < 0.05).
TRAF2 is essential for CTAR1-mediated activation of NF-κB signaling, while TRAF6 is a crucial intermediate regulating the ability of CTAR2 to activate the canonical NF-κB pathway (3, 8, 9, 45). It was therefore hypothesized that canonical TRAF6-mediated NF-κB signaling would be required for LMP1 to activate IGF1R. To test this theory, an IκBα superrepressor (SR) was used to inhibit canonical NF-κB signaling as previously described (46, 47). Briefly, degradation of IκBα is required for nuclear localization of NF-κB canonical transcription factors. Mutation of serine residues within IκBα prevents its degradation and inhibits NF-κB signaling (47). Moreover, the IκBα SR has specifically been shown to be effective at preventing TRAF-mediated NF-κB activation (30, 46). A dominant negative TRAF6 construct was used to impede TRAF6 function as described previously (5, 31). CTAR1 activation of NF-κB signaling was inhibited using a myc-tagged dominant negative TRAF2 construct that has been described previously (5, 32). FLAG-HA-tagged LMP1 was transfected into cells with and without the HA-tagged SR, myc-tagged dnTRAF2, or dnTRAF6. The resulting lysates were probed for LMP1, IκBα, Myc, and TRAF6 to verify that all constructs were expressed as expected (Fig. 6E). Importantly, IκBα SR, dnTRAF2, and dnTRAF6 failed to influence the overall expression of IGF1R (Fig. 6E). Two days after transfection, cell lysates were immunoprecipitated with anti-pTyr and probed with anti-IGF1R to specifically detect pIGF1R. Expression of the SR and dnTRAF6 selectively decreased the activation of IGF1R to levels equivalent to those for the pBabe vector control, while dnTRAF2 did not decrease pIGF1R levels relative to those for LMP1 (Fig. 6F). In agreement with these data, qRT-PCR analysis indicated that the presence of dnTRAF2 did not impact the ability of LMP1 to induce IGF1 mRNA relative to pBabe vector controls (Fig. 6G). In contrast, the presence of the SR or dnTRAF6 inhibited IGF1 expression relative to that in pBabe control cells (Fig. 6G). The expression of LMP1 with or without the SR, dnTRAF2, or dnTRAF6 did not alter the transcription of IGF1R (Fig. 6G). In summary, these findings reveal that LMP1 activates the canonical NF-κB pathway to increase expression of IGF1 and activate IGF1R.
DISCUSSION
The transforming properties of LMP1 have been described in multiple systems. It is thought that the effects of the LMP1 CTAR1 domain on EGFR expression are important components in altering epithelial cell growth. LMP1 increases EGFR expression through an unusual form of NF-κB signaling mediated by p50 and BCL3 (10, 15–17). Similarly, NF-κB signaling may also induce the expression of the RTK RON (26, 27). Analysis of 293T cells transfected with LMP1 or pBabe controls did not reveal differential RON phosphorylation, but EGFR activation was enhanced, in keeping with previous results (Fig. 1A to C). Interestingly, LMP1 selectively increased the phosphorylation of IGF1R, and this effect was validated in multiple cell lines regardless of whether LMP1 was transiently or stably expressed (Fig. 1C and D). EBV infects both epithelial cells and B cells, and LMP1 may differentially regulate RTKs depending on the cellular context. For instance, LMP1 induces RON expression in B cells and NPC cells, while induction of EGFR expression occurs primarily in epithelial cells (15–17, 26, 27). Previous analyses of lymphocytes suggested that EBV infection decreased the expression of IGF1R, yet the data presented in this paper indicate that LMP1 increases IGF1R activation in fibroblasts and immortalized or transformed epithelial cells (48) (Fig. 1). These data suggest that LMP1 uses different mechanisms to regulate IGF1R expression and activation in lymphocytes and in epithelial cells. In addition to showing that LMP1 selectively activated IGF1R, these studies revealed that targeting IGF1R with small-molecule inhibitors or with shRNA specifically impaired the Akt phosphorylation, proliferation, and focus formation of LMP1-positive cells (Fig. 2, 3C to E, and 4). In keeping with previous findings, PPP targeted IGF1R with an IC50 of approximately 50 nM (39). In this study, AG1024 targeted IGF1R with an IC50 of roughly 20 μM, which is higher than the IC50 of 7 μM reported by other groups. Nevertheless, the 20 μM IC50 value observed for IGF1R is still below the 57 μM IC50 at which AG1024 inhibits IR, indicating that AG1024 would maintain specificity for IGF1R at a concentration of 20 μM (40). It should be noted that AG 1024 targets IGF1R without influencing EGFR activation at concentrations up to 70 μM and PPP does not alter pEGFR levels at doses below 1 μM (49, 50). The concentrations of IGF1R inhibitors used in this study would not directly affect EGFR activation, indicating that the effects detected after treatment with AG1024 and PPP reflect effects on IGF1R phosphorylation.
Since PPP, AG1024, and shRNA against IGF1R all generated similar phenotypes in LMP1-expressing cells, it is likely that the effects generated by the small-molecule inhibitors are specifically due to inhibition of IGF1R (Fig. 2 to 4). Inhibiting IGF1R reduced both proliferation and Akt activation in 293T, MCF10a, and Rat1 cells, indicating that LMP1 expression, rather than cell type, imposes a requirement for active IGF1R signaling (Fig. 2 to 4). Constitutively active myr-Akt rescued the decreased proliferation detected in LMP1-positive cells upon IGF1R inhibition, suggesting that IGF1R-mediated phosphorylation of Akt is required for cell growth (Fig. 3F and G). Interestingly, inhibiting IGF1R does not impair the migratory effects induced by LMP1 expression in rodent fibroblasts, suggesting that LMP1-mediated activation of IGF1R primarily induces cell proliferation (Fig. 3A and B).
IGF1R activating mutations are rare, and to date, no such mutations have been reported in cancer. In transformed cells, IGF1R is frequently activated by overexpression or by alterations in ligand expression or secretion. Although both IGF1 and IGF2 transcription may be altered, changes in IGF2 expression are more frequently detected in cancer (51). LMP1 did not modify IGF1R or IGF2 expression, but it did increase both the mRNA expression and secretion of IGF1 by 1.5- to 2-fold (Fig. 5A and B). Treatment with an anti-IGF1R designed to prevent ligand binding reduced the activation of IGF1R and selectively decreased the proliferation of LMP1-positive cells relative to vector control cells, thus reinforcing the finding that LMP1 regulates IGF1R activity in a ligand-dependent manner (Fig. 5C and D). Interestingly, anti-IGF1R did not reduce LMP1-positive cell proliferation as much as targeting IGF1R with small-molecule inhibitors or shRNA (Fig. 2C and D, 4C, and 5D). It may be that anti-IGF1R does not decrease IGF1R activation to the same extent as the other methodologies or that there are other, as-yet-unidentified LMP1-dependent mechanisms that promote IGF1R activation. It has been shown that the EBV-encoded small RNAs (EBERs) can induce IGF1 secretion (52). It is therefore possible that EBERs may cooperate with LMP1 to activate IGF1R during epithelial infection.
LMP1 has two signaling domains, CTAR1 and CTAR2, which regulate a large portion of the signaling capabilities of LMP1. The LMP1 378 mutation targeting CTAR2 selectively reduced the mRNA expression and secretion of IGF1 relative to that for WT LMP1 and the LMP1 A5 mutant targeting CTAR1 (Fig. 6C and D). In keeping with these findings, LMP1 A5 did not alter IGF1R phosphorylation relative to that for WT LMP1, while LMP1 378 reduced IGF1R phosphorylation to levels equivalent to those in pBabe control cells (Fig. 6A and B). The CTAR2 domain of LMP1 is known to activate canonical NF-κB signaling via TRAF6, and it was hypothesized that both TRAF6 and NF-κB were required for LMP1 to increase IGF1 mRNA levels and IGF1R activation (9, 46, 47). Indeed, inhibition of NF-κB with the IκBα SR and inhibition of TRAF6 signaling with dnTRAF6 both reduced IGF1R activation and IGF1 synthesis (Fig. 6E to G). The specificity of these observations was confirmed by the fact that expression of dnTRAF2 did not impact IGF1 mRNA expression or pIGF1R levels (Fig. 6E to G). Interestingly, neither the SR nor dnTRAF6 impacted the expression of IGF1R (Fig. 6G).
It has been shown that NPC tumors express both EGFR and IGF1R, although it is not clear whether IGF1R expression is specifically upregulated in EBV-infected tumors (53–55). High expression of IGF1R has been correlated with poor NPC patient survival, and targeting NPC xenografts with PPP reduces tumor growth (53, 56). Additionally, preliminary data suggest that targeting both EGFR and IGF1R in NPC may be more beneficial than targeting either RTK individually (15, 57). The work described in this paper reveals that LMP1 activates not only EGFR but also IGF1R. These findings may provide the underlying mechanism behind the effects described in the IGF1R-directed clinical studies. Importantly, these clinical reports support the concept that IGF1R is a viable therapeutic target in NPC patients and that targeting both EGFR and IGF1R may be more beneficial than targeting either RTK individually.
IGF1R is capable of interacting with other RTKs to promote cross-phosphorylation and downstream signaling. Of note, IGF1R can physically associate with EGFR, and the ligands EGF and IGF1 can activate both EGFR and IGF1R even when the two receptors are not physically connected (58–61). Since LMP1 regulates activation of EGFR and IGF1R, it is possible that LMP1 promotes interaction between these receptors to facilitate their phosphorylation. Alternately, although it is known that LMP1 increases EGFR phosphorylation, it is not yet known how this elevated activation occurs. Perhaps the increased secretion of IGF1 induced by LMP1 is responsible for promoting EGFR phosphorylation. To facilitate the use of EGFR and IGF1R as therapeutic targets, it will be important to explore the potential interaction and cross-phosphorylation between these two RTKs.
In summary, the data in this paper demonstrate that the CTAR2 domain of LMP1 signals through the canonical NF-κB pathway to increase the mRNA expression and secretion of the IGF1R ligand IGF1. The elevated secretion of IGF1 promotes the phosphorylation of IGF1R, leading to the phosphorylation of Akt and increased cell proliferation. Inhibition of IGF1R selectively reduces the Akt phosphorylation, cell proliferation, and focus formation of LMP1-positive cells. These data describe a novel mechanism through which LMP1 promotes cellular transformation and also identify a potential method to selectively target EBV-positive, LMP1-expressing cells. Taken together, these findings may yield improved therapeutics for the treatment of NPC.
ACKNOWLEDGMENTS
This work was supported by NIH grants CA32979 and 19014 to N.R-T. K.T. received support from grant T32CA009156.
We thank Anuja Mathur for technical assistance.
We declare no conflicts of interest.
REFERENCES
- 1.Raab-Traub N. 2007. EBV-induced oncogenesis, p 986–1006 InArvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom. [PubMed] [Google Scholar]
- 2.Cohen JI, Mocarski ES, Raab-Traub N, Corey L, Nabel GJ. 2013. The need and challenges for development of an Epstein-Barr virus vaccine. Vaccine 31(Suppl 2):B194–B196. doi: 10.1016/j.vaccine.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson CW, Port RJ, Young LS. 2012. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol 22:144–153. doi: 10.1016/j.semcancer.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Devergne O, Cahir McFarland ED, Mosialos G, Izumi KM, Ware CF, Kieff E. 1998. Role of the TRAF binding site and NF-kappaB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J Virol 72:7900–7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mainou BA, Everly DN Jr, Raab-Traub N. 2007. Unique signaling properties of CTAR1 in LMP1-mediated transformation. J Virol 81:9680–9692. doi: 10.1128/JVI.01001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izumi KM, Kieff ED. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-kappaB. Proc Natl Acad Sci U S A 94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito N, Courtois G, Chiba A, Yamamoto N, Nitta T, Hironaka N, Rowe M, Yamaoka S. 2003. Two carboxyl-terminal activation regions of Epstein-Barr virus latent membrane protein 1 activate NF-kappaB through distinct signaling pathways in fibroblast cell lines. J Biol Chem 278:46565–46575. doi: 10.1074/jbc.M302549200. [DOI] [PubMed] [Google Scholar]
- 8.Luftig M, Yasui T, Soni V, Kang MS, Jacobson N, Cahir-McFarland E, Seed B, Kieff E. 2004. Epstein-Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK alpha-dependent noncanonical NF-kappaB activation. Proc Natl Acad Sci U S A 101:141–146. doi: 10.1073/pnas.2237183100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soni V, Cahir-McFarland E, Kieff E. 2007. LMP1 TRAFficking activates growth and survival pathways. Adv Exp Med Biol 597:173–187. doi: 10.1007/978-0-387-70630-6_14. [DOI] [PubMed] [Google Scholar]
- 10.Thornburg NJ, Pathmanathan R, Raab-Traub N. 2003. Activation of nuclear factor-kappaB p50 homodimer/Bcl-3 complexes in nasopharyngeal carcinoma. Cancer Res 63:8293–8301. [PubMed] [Google Scholar]
- 11.Paine E, Scheinman RI, Baldwin AS Jr, Raab-Traub N. 1995. Expression of LMP1 in epithelial cells leads to the activation of a select subset of NF-kappa B/Rel family proteins. J Virol 69:4572–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thornburg NJ, Raab-Traub N. 2007. Induction of epidermal growth factor receptor expression by Epstein-Barr virus latent membrane protein 1 C-terminal-activating region 1 is mediated by NF-kappaB p50 homodimer/Bcl-3 complexes. J Virol 81:12954–12961. doi: 10.1128/JVI.01601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie P. 2013. TRAF molecules in cell signaling and in human diseases. J Mol Signal 8:7. doi: 10.1186/1750-2187-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Wang Y, Zeng S, Hu X. 2012. LMP1 expression is positively associated with metastasis of nasopharyngeal carcinoma: evidence from a meta-analysis. J Clin Pathol 65:41–45. doi: 10.1136/jclinpath-2011-200198. [DOI] [PubMed] [Google Scholar]
- 15.Miller WE, Earp HS, Raab-Traub N. 1995. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J Virol 69:4390–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kung CP, Raab-Traub N. 2008. Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor through effects on Bcl-3 and STAT3. J Virol 82:5486–5493. doi: 10.1128/JVI.00125-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller WE, Cheshire JL, Raab-Traub N. 1998. Interaction of tumor necrosis factor receptor-associated factor signaling proteins with the latent membrane protein 1 PXQXT motif is essential for induction of epidermal growth factor receptor expression. Mol Cell Biol 18:2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kung CP, Meckes DG Jr, Raab-Traub N. 2011. Epstein-Barr virus LMP1 activates EGFR, STAT3, and ERK through effects on PKCdelta. J Virol 85:4399–4408. doi: 10.1128/JVI.01703-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson D, Charalambous C, Wilson JB. 2005. Epstein-Barr virus latent membrane protein 1 (CAO) up-regulates VEGF and TGF alpha concomitant with hyperlasia, with subsequent up-regulation of p16 and MMP9. Cancer Res 65:8826–8835. doi: 10.1158/0008-5472.CAN-05-0591. [DOI] [PubMed] [Google Scholar]
- 20.Lemmon MA, Schlessinger J. 2010. Cell signaling by receptor tyrosine kinases. Cell 141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lui VW, Lau CP, Ho K, Ng MH, Cheng SH, Tsao SW, Tsang CM, Lei KI, Chan AT, Mok TS. 2011. Anti-invasion, anti-proliferation and anoikis-sensitization activities of lapatinib in nasopharyngeal carcinoma cells. Invest New Drugs 29:1241–1252. doi: 10.1007/s10637-010-9470-y. [DOI] [PubMed] [Google Scholar]
- 22.Xiao X, Wu J, Zhu X, Zhao P, Zhou J, Liu QQ, Zheng L, Zeng M, Liu R, Huang W. 2007. Induction of cell cycle arrest and apoptosis in human nasopharyngeal carcinoma cells by ZD6474, an inhibitor of VEGFR tyrosine kinase with additional activity against EGFR tyrosine kinase. Int J Cancer 121:2095–2104. doi: 10.1002/ijc.22955. [DOI] [PubMed] [Google Scholar]
- 23.Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. 2012. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets 16:15–31. doi: 10.1517/14728222.2011.648617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luraghi P, Reato G, Cipriano E, Sassi F, Orzan F, Bigatto V, De Bacco F, Menietti E, Han M, Rideout WM III, Perera T, Bertotti A, Trusolino L, Comoglio PM, Boccaccio C. 2014. MET signaling in colon cancer stem-like cells blunts the therapeutic response to EGFR inhibitors. Cancer Res 74:1857–1869. doi: 10.1158/0008-5472.CAN-13-2340-T. [DOI] [PubMed] [Google Scholar]
- 25.Han W, Lo HW. 2012. Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett 318:124–134. doi: 10.1016/j.canlet.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou YC, Lin SJ, Lu J, Yeh TH, Chen CL, Weng PL, Lin JH, Yao M, Tsai CH. 2011. Requirement for LMP1-induced RON receptor tyrosine kinase in Epstein-Barr virus-mediated B-cell proliferation. Blood 118:1340–1349. doi: 10.1182/blood-2011-02-335448. [DOI] [PubMed] [Google Scholar]
- 27.Chou YC, Chen CL, Yeh TH, Lin SJ, Chen MR, Doong SL, Lu J, Tsai CH. 2012. Involvement of recepteur d'origine nantais receptor tyrosine kinase in Epstein-Barr virus-associated nasopharyngeal carcinoma and its metastasis. Am J Pathol 181:1773–1781. doi: 10.1016/j.ajpath.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 29.Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. 1999. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A 96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shair KH, Schnegg CI, Raab-Traub N. 2009. Epstein-Barr virus latent membrane protein-1 effects on junctional plakoglobin and induction of a cadherin switch. Cancer Res 69:5734–5742. doi: 10.1158/0008-5472.CAN-09-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciencewicki J, Brighton L, Wu WD, Madden M, Jaspers I. 2006. Diesel exhaust enhances virus- and poly(I:C)-induced Toll-like receptor 3 expression and signaling in respiratory epithelial cells. Am J Physiol Lung Cell Mol Physiol 290:L1154–L1163. doi: 10.1152/ajplung.00318.2005. [DOI] [PubMed] [Google Scholar]
- 32.Takeshita H, Yoshizaki T, Miller WE, Sato H, Furukawa M, Pagano JS, Raab-Traub N. 1999. Matrix metalloproteinase 9 expression is induced by Epstein-Barr virus latent membrane protein 1 C-terminal activation regions 1 and 2. J Virol 73:5548–5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meckes DG Jr, Menaker NF, Raab-Traub N. 2013. Epstein-Barr virus LMP1 modulates lipid raft microdomains and the vimentin cytoskeleton for signal transduction and transformation. J Virol 87:1301–1311. doi: 10.1128/JVI.02519-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller WE, Cheshire JL, Baldwin AS Jr, Raab-Traub N. 1998. The NPC derived C15 LMP1 protein confers enhanced activation of NF-kappa B and induction of the EGFR in epithelial cells. Oncogene 16:1869–1877. doi: 10.1038/sj.onc.1201696. [DOI] [PubMed] [Google Scholar]
- 36.Cheung ST, Huang DP, Hui AB, Lo KW, Ko CW, Tsang YS, Wong N, Whitney BM, Lee JC. 1999. Nasopharyngeal carcinoma cell line (C666-1) consistently harbouring Epstein-Barr virus. Int J Cancer 83:121–126. doi:. [DOI] [PubMed] [Google Scholar]
- 37.Girnita L, Worrall C, Takahashi S, Seregard S, Girnita A. 2014. Something old, something new and something borrowed: emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell Mol Life Sci 71:2403–2427. doi: 10.1007/s00018-013-1514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li R, Pourpak A, Morris SW. 2009. Inhibition of the insulin-like growth factor-1 receptor (IGF1R) tyrosine kinase as a novel cancer therapy approach. J Med Chem 52:4981–5004. doi: 10.1021/jm9002395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Girnita A, All-Ericsson C, Economou MA, Astrom K, Axelson M, Seregard S, Larsson O, Girnita L. 2006. The insulin-like growth factor-I receptor inhibitor picropodophyllin causes tumor regression and attenuates mechanisms involved in invasion of uveal melanoma cells. Clin Cancer Res 12:1383–1391. doi: 10.1158/1078-0432.CCR-05-1106. [DOI] [PubMed] [Google Scholar]
- 40.Parrizas M, Gazit A, Levitzki A, Wertheimer E, LeRoith D. 1997. Specific inhibition of insulin-like growth factor-1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins. Endocrinology 138:1427–1433. doi: 10.1210/en.138.4.1427. [DOI] [PubMed] [Google Scholar]
- 41.Jones HE, Goddard L, Gee JM, Hiscox S, Rubini M, Barrow D, Knowlden JM, Williams S, Wakeling AE, Nicholson RI. 2004. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer 11:793–814. doi: 10.1677/erc.1.00799. [DOI] [PubMed] [Google Scholar]
- 42.Yaktapour N, Ubelhart R, Schuler J, Aumann K, Dierks C, Burger M, Pfeifer D, Jumaa H, Veelken H, Brummer T, Zirlik K. 2013. Insulin-like growth factor-1 receptor (IGF1R) as a novel target in chronic lymphocytic leukemia. Blood 122:1621–1633. doi: 10.1182/blood-2013-02-484386. [DOI] [PubMed] [Google Scholar]
- 43.Xiao L, Hu ZY, Dong X, Tan Z, Li W, Tang M, Chen L, Yang L, Tao Y, Jiang Y, Li J, Yi B, Li B, Fan S, You S, Deng X, Hu F, Feng L, Bode AM, Dong Z, Sun LQ, Cao Y. 2014. Targeting Epstein-Barr virus oncoprotein LMP1-mediated glycolysis sensitizes nasopharyngeal carcinoma to radiation therapy. Oncogene 33:4568–4578. doi: 10.1038/onc.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen CC, Liu HP, Chao M, Liang Y, Tsang NM, Huang HY, Wu CC, Chang YS. 2014. NF-kappaB-mediated transcriptional upregulation of TNFAIP2 by the Epstein-Barr virus oncoprotein, LMP1, promotes cell motility in nasopharyngeal carcinoma. Oncogene 33:3648–3659. doi: 10.1038/onc.2013.345. [DOI] [PubMed] [Google Scholar]
- 45.Kung CP, Raab-Traub N. 2010. Epstein-Barr virus latent membrane protein 1 modulates distinctive NF-kappaB pathways through C-terminus-activating region 1 to regulate epidermal growth factor receptor expression. J Virol 84:6605–6614. doi: 10.1128/JVI.00344-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Natoli G, Costanzo A, Guido F, Moretti F, Bernardo A, Burgio VL, Agresti C, Levrero M. 1998. Nuclear factor kB-independent cytoprotective pathways originating at tumor necrosis factor receptor-associated factor 2. J Biol Chem 273:31262–31272. doi: 10.1074/jbc.273.47.31262. [DOI] [PubMed] [Google Scholar]
- 47.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. 1996. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol 16:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kriauciunas KM, Goldstein BJ, Lipes MA, Kahn CR. 1993. Modulation of expression of insulin and IGF-I receptor by Epstein-Barr virus and its gene products LMP and EBNA-2 in lymphocyte cell lines. J Cell Physiol 154:486–495. doi: 10.1002/jcp.1041540306. [DOI] [PubMed] [Google Scholar]
- 49.Li P, Veldwijk MR, Zhang Q, Li ZB, Xu WC, Fu S. 2013. Co-inhibition of epidermal growth factor receptor and insulin-like growth factor receptor 1 enhances radiosensitivity in human breast cancer cells. BMC Cancer 13:297. doi: 10.1186/1471-2407-13-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu X, Sooman L, Wickstrom M, Fryknas M, Dyrager C, Lennartsson J, Gullbo J. 2013. Alternative cytotoxic effects of the postulated IGF-IR inhibitor picropodophyllin in vitro. Mol Cancer Ther 12:1526–1536. doi: 10.1158/1535-7163.MCT-13-0091. [DOI] [PubMed] [Google Scholar]
- 51.Werner H, Roberts CT Jr. 2003. The IGFI receptor gene: a molecular target for disrupted transcription factors. Genes Chromosomes Cancer 36:113–120. doi: 10.1002/gcc.10157. [DOI] [PubMed] [Google Scholar]
- 52.Iwakiri D, Sheen TS, Chen JY, Huang DP, Takada K. 2005. Epstein-Barr virus-encoded small RNA induces insulin-like growth factor 1 and supports growth of nasopharyngeal carcinoma-derived cell lines. Oncogene 24:1767–1773. doi: 10.1038/sj.onc.1208357. [DOI] [PubMed] [Google Scholar]
- 53.Yuan Y, Zhou X, Song J, Qiu X, Li J, Ye L, Meng X, Xia D. 2008. Expression and clinical significance of epidermal growth factor receptor and type 1 insulin-like growth factor receptor in nasopharyngeal carcinoma. Ann Otol Rhinol Laryngol 117:192–200. [DOI] [PubMed] [Google Scholar]
- 54.Friedrich RE, Hagel C, Bartel-Friedrich S. 2010. Insulin-like growth factor-1 receptor (IGF-1R) in primary and metastatic undifferentiated carcinoma of the head and neck: a possible target of immunotherapy. Anticancer Res 30:1641–1643. [PubMed] [Google Scholar]
- 55.Yip WK, Leong VC, Abdullah MA, Yusoff S, Seow HF. 2008. Overexpression of phospho-Akt correlates with phosphorylation of EGF receptor, FKHR and BAD in nasopharyngeal carcinoma. Oncol Rep 19:319–328. doi: 10.3892/or.19.2.319. [DOI] [PubMed] [Google Scholar]
- 56.Yin SC, Guo W, Tao ZZ. 2013. Picropodophyllin inhibits tumor growth of human nasopharyngeal carcinoma in a mouse model. Biochem Biophys Res Commun 439:1–5. doi: 10.1016/j.bbrc.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 57.Yuan YL, Zhou XH, Song J, Qiu XP, Li J, Ye LF. 2008. Dual silencing of type 1 insulin-like growth factor and epidermal growth factor receptors to induce apoptosis of nasopharyngeal cancer cells. J Laryngol Otol 122:952–960. doi: 10.1017/S0022215107000606. [DOI] [PubMed] [Google Scholar]
- 58.Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. 2000. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J Biol Chem 275:22583–22589. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- 59.Hallak H, Moehren G, Tang J, Kaou M, Addas M, Hoek JB, Rubin R. 2002. Epidermal growth factor-induced activation of the insulin-like growth factor I receptor in rat hepatocytes. Hepatology 36:1509–1518. doi: 10.1053/jhep.2002.37138. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Yuan JL, Zhang YT, Ma JJ, Xu P, Shi CH, Zhang W, Li YM, Fu Q, Zhu GF, Xue W, Lei YH, Gao JY, Wang JY, Shao C, Yi CG, Wang H. 2013. Inhibition of both EGFR and IGF1R sensitized prostate cancer cells to radiation by synergistic suppression of DNA homologous recombination repair. PLoS One 8:e68784. doi: 10.1371/journal.pone.0068784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riedemann J, Takiguchi M, Sohail M, Macaulay VM. 2007. The EGF receptor interacts with the type 1 IGF receptor and regulates its stability. Biochem Biophys Res Commun 355:707–714. doi: 10.1016/j.bbrc.2007.02.012. [DOI] [PubMed] [Google Scholar]